Adult Human Brain Tissue Cultures to Study NeuroHIV
- PMID: 38994979
- PMCID: PMC11240386
- DOI: 10.3390/cells13131127
Adult Human Brain Tissue Cultures to Study NeuroHIV
Abstract
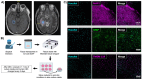
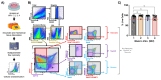
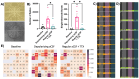
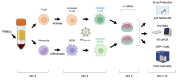
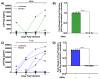
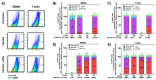
HIV-associated neurocognitive disorders (HAND) persist under antiretroviral therapy as a complex pathology that has been difficult to study in cellular and animal models. Therefore, we generated an ex vivo human brain slice model of HIV-1 infection from surgically resected adult brain tissue. Brain slice cultures processed for flow cytometry showed >90% viability of dissociated cells within the first three weeks in vitro, with parallel detection of astrocyte, myeloid, and neuronal populations. Neurons within brain slices showed stable dendritic spine density and mature spine morphologies in the first weeks in culture, and they generated detectable activity in multi-electrode arrays. We infected cultured brain slices using patient-matched CD4+ T-cells or monocyte-derived macrophages (MDMs) that were exposed to a GFP-expressing R5-tropic HIV-1 in vitro. Infected slice cultures expressed viral RNA and developed a spreading infection up to 9 days post-infection, which were significantly decreased by antiretrovirals. We also detected infected myeloid cells and astrocytes within slices and observed minimal effect on cellular viability over time. Overall, this human-centered model offers a promising resource to study the cellular mechanisms contributing to HAND (including antiretroviral toxicity, substance use, and aging), infection of resident brain cells, and new neuroprotective therapeutics.
Keywords: HIV-1 infection; HIV-associated neurocognitive disorders (HAND); dendritic spine analysis; human brain organotypic slice cultures; microelectrode array.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. (Author Atom Sarkar was employed by the company Global Neurosciences Institute, LLC. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.)
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

