Inhibition of Vascular Smooth Muscle Cell Proliferation by ENPP1: The Role of CD73 and the Adenosine Signaling Axis
- PMID: 38994980
- PMCID: PMC11240470
- DOI: 10.3390/cells13131128
Inhibition of Vascular Smooth Muscle Cell Proliferation by ENPP1: The Role of CD73 and the Adenosine Signaling Axis
Abstract
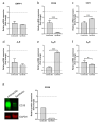
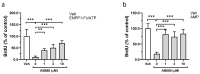
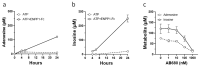
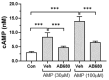
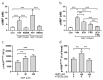
The Ectonucleotide Pyrophosphatase/Phosphodiesterase 1 (ENPP1) ectoenzyme regulates vascular intimal proliferation and mineralization of bone and soft tissues. ENPP1 variants cause Generalized Arterial Calcification of Infancy (GACI), a rare genetic disorder characterized by ectopic calcification, intimal proliferation, and stenosis of large- and medium-sized arteries. ENPP1 hydrolyzes extracellular ATP to pyrophosphate (PPi) and AMP. AMP is the precursor of adenosine, which has been implicated in the control of neointimal formation. Herein, we demonstrate that an ENPP1-Fc recombinant therapeutic inhibits proliferation of vascular smooth muscle cells (VSMCs) in vitro and in vivo. Addition of ENPP1 and ATP to cultured VSMCs generated AMP, which was metabolized to adenosine. It also significantly decreased cell proliferation. AMP or adenosine alone inhibited VSMC growth. Inhibition of ecto-5'-nucleotidase CD73 decreased adenosine accumulation and suppressed the anti-proliferative effects of ENPP1/ATP. Addition of AMP increased cAMP synthesis and phosphorylation of VASP at Ser157. This AMP-mediated cAMP increase was abrogated by CD73 inhibitors or by A2aR and A2bR antagonists. Ligation of the carotid artery promoted neointimal hyperplasia in wild-type mice, which was exacerbated in ENPP1-deficient ttw/ttw mice. Prophylactic or therapeutic treatments with ENPP1 significantly reduced intimal hyperplasia not only in ttw/ttw but also in wild-type mice. These findings provide the first insight into the mechanism of the anti-proliferative effect of ENPP1 and broaden its potential therapeutic applications beyond enzyme replacement therapy.
Keywords: neointima; purinergic signaling; vascular smooth muscle cells.
Conflict of interest statement
B.T., D.C., C.S., L.F., K.O., J.H., D.T., D.O. and Y.S. are current employees and stockholders of Inozyme Pharma. Z.C. was an employee of Inozyme Pharma at the time of the study. F.R. is stockholder of Inozyme Pharma and received consulting fees from Inozyme Pharma. The remaining authors declare that they have no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

