In Vivo Monitoring of Fabp7 Expression in Transgenic Zebrafish
- PMID: 38994990
- PMCID: PMC11240397
- DOI: 10.3390/cells13131138
In Vivo Monitoring of Fabp7 Expression in Transgenic Zebrafish
Abstract
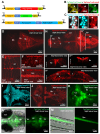
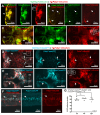
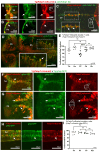
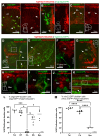
In zebrafish, like in mammals, radial glial cells (RGCs) can act as neural progenitors during development and regeneration in adults. However, the heterogeneity of glia subpopulations entails the need for different specific markers of zebrafish glia. Currently, fluorescent protein expression mediated by a regulatory element from the glial fibrillary acidic protein (gfap) gene is used as a prominent glia reporter. We now expand this tool by demonstrating that a regulatory element from the mouse Fatty acid binding protein 7 (Fabp7) gene drives reliable expression in fabp7-expressing zebrafish glial cells. By using three different Fabp7 regulatory element-mediated fluorescent protein reporter strains, we reveal in double transgenic zebrafish that progenitor cells expressing fluorescent proteins driven by the Fabp7 regulatory element give rise to radial glia, oligodendrocyte progenitors, and some neuronal precursors. Furthermore, Bergmann glia represent the almost only glial population of the zebrafish cerebellum (besides a few oligodendrocytes), and the radial glia also remain in the mature cerebellum. Fabp7 regulatory element-mediated reporter protein expression in Bergmann glia progenitors suggests their origin from the ventral cerebellar proliferation zone, the ventricular zone, but not from the dorsally positioned upper rhombic lip. These new Fabp7 reporters will be valuable for functional studies during development and regeneration.
Keywords: Bergmann glia; Fabp7; cell progenitors; cerebellum; neurons; oligodendrocytes; radial glia; transgenic line; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum.Cell Death Dis. 2013 Mar 14;4(3):e546. doi: 10.1038/cddis.2013.74. Cell Death Dis. 2013. PMID: 23492777 Free PMC article.
-
Gfap-positive radial glial cells are an essential progenitor population for later-born neurons and glia in the zebrafish spinal cord.Glia. 2016 Jul;64(7):1170-89. doi: 10.1002/glia.22990. Epub 2016 Apr 21. Glia. 2016. PMID: 27100776 Free PMC article.
-
Notch-regulated oligodendrocyte specification from radial glia in the spinal cord of zebrafish embryos.Dev Dyn. 2008 Aug;237(8):2081-9. doi: 10.1002/dvdy.21620. Dev Dyn. 2008. PMID: 18627107 Free PMC article.
-
Macrophage-Neuroglia Interactions in Promoting Neuronal Regeneration in Zebrafish.Int J Mol Sci. 2023 Mar 30;24(7):6483. doi: 10.3390/ijms24076483. Int J Mol Sci. 2023. PMID: 37047456 Free PMC article. Review.
-
Origin, lineage and function of cerebellar glia.Prog Neurobiol. 2013 Oct;109:42-63. doi: 10.1016/j.pneurobio.2013.08.001. Epub 2013 Aug 25. Prog Neurobiol. 2013. PMID: 23981535 Review.
Cited by
-
A single-cell landscape of the regenerating spinal cord of zebrafish.Neural Regen Res. 2026 Feb 1;21(2):780-789. doi: 10.4103/NRR.NRR-D-24-01163. Epub 2025 Apr 30. Neural Regen Res. 2026. PMID: 40326988 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

