Iron Regulates Cellular Proliferation by Enhancing the Expression of Glucose Transporter GLUT3 in the Liver
- PMID: 38994998
- PMCID: PMC11240476
- DOI: 10.3390/cells13131147
Iron Regulates Cellular Proliferation by Enhancing the Expression of Glucose Transporter GLUT3 in the Liver
Abstract
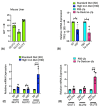
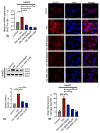
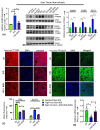
Iron is often accumulated in the liver during pathological conditions such as cirrhosis and cancer. Elevated expression of glucose transporters GLUT1 and GLUT3 is associated with reduced overall survival in patients with hepatocellular carcinoma. However, it is not known whether iron can regulate glucose transporters and contribute to tumor proliferation. In the present study, we found that treatment of human liver cell line HepG2 with ferric ammonium citrate (FAC) resulted in a significant upregulation of GLUT3 mRNA and protein in a dose-dependent manner. Similarly, iron accumulation in mice fed with high dietary iron as well as in mice injected intraperitoneally with iron dextran enhanced the GLUT3 expression drastically in the liver. We demonstrated that iron-induced hepatic GLUT3 upregulation is mediated by the LKB1/AMPK/CREB1 pathway, and this activation was reversed when treated with iron chelator deferiprone. In addition, inhibition of GLUT3 using siRNA prevented iron-mediated increase in the expression of cell cycle markers and cellular hyperproliferation. Furthermore, exogenous sodium beta-hydroxybutyrate treatment prevented iron-mediated hepatic GLUT3 activation both in vitro and in vivo. Together, these results underscore the importance of iron, AMPK, CREB1 and GLUT3 pathways in cell proliferation and highlight the therapeutic potential of sodium beta-hydroxybutyrate in hepatocellular carcinoma with high GLUT3 expression.
Keywords: AMPK; CREB1; GLUT3; cell proliferation; iron overload.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

