Regeneration of Propriospinal Axons in Rat Transected Spinal Cord Injury through a Growth-Promoting Pathway Constructed by Schwann Cells Overexpressing GDNF
- PMID: 38995011
- PMCID: PMC11240522
- DOI: 10.3390/cells13131160
Regeneration of Propriospinal Axons in Rat Transected Spinal Cord Injury through a Growth-Promoting Pathway Constructed by Schwann Cells Overexpressing GDNF
Abstract
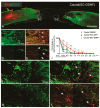
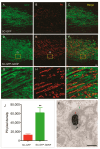
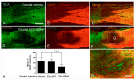
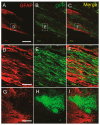
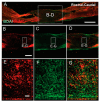
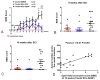
Unsuccessful axonal regeneration in transected spinal cord injury (SCI) is mainly attributed to shortage of growth factors, inhibitory glial scar, and low intrinsic regenerating capacity of severely injured neurons. Previously, we constructed an axonal growth permissive pathway in a thoracic hemisected injury by transplantation of Schwann cells overexpressing glial-cell-derived neurotrophic factor (SCs-GDNF) into the lesion gap as well as the caudal cord and proved that this novel permissive bridge promoted the regeneration of descending propriospinal tract (dPST) axons across and beyond the lesion. In the current study, we subjected rats to complete thoracic (T11) spinal cord transections and examined whether these combinatorial treatments can support dPST axons' regeneration beyond the transected injury. The results indicated that GDNF significantly improved graft-host interface by promoting integration between SCs and astrocytes, especially the migration of reactive astrocyte into SCs-GDNF territory. The glial response in the caudal graft area has been significantly attenuated. The astrocytes inside the grafted area were morphologically characterized by elongated and slim process and bipolar orientation accompanied by dramatically reduced expression of glial fibrillary acidic protein. Tremendous dPST axons have been found to regenerate across the lesion and back to the caudal spinal cord which were otherwise difficult to see in control groups. The caudal synaptic connections were formed, and regenerated axons were remyelinated. The hindlimb locomotor function has been improved.
Keywords: Schwann cell; descending propriospinal axon; functional recovery; glial response; glial scar; glial-cell-derived neurotrophic factor; regeneration; spinal cord injury; spinal transection; transplantation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

