Tumor Necrosis Factor-Alpha Modulates Expression of Genes Involved in Cytokines and Chemokine Pathways in Proliferative Myoblast Cells
- PMID: 38995013
- PMCID: PMC11240656
- DOI: 10.3390/cells13131161
Tumor Necrosis Factor-Alpha Modulates Expression of Genes Involved in Cytokines and Chemokine Pathways in Proliferative Myoblast Cells
Abstract
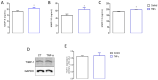
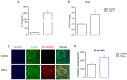
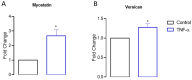
Skeletal muscle regeneration after injury is a complex process involving inflammatory signaling and myoblast activation. Pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) are key mediators, but their effects on gene expression in proliferating myoblasts are unclear. We performed the RNA sequencing of TNF-α treated C2C12 myoblasts to elucidate the signaling pathways and gene networks regulated by TNF-α during myoblast proliferation. The TNF-α (10 ng/mL) treatment of C2C12 cells led to 958 differentially expressed genes compared to the controls. Pathway analysis revealed significant regulation of TNF-α signaling, along with the chemokine and IL-17 pathways. Key upregulated genes included cytokines (e.g., IL-6), chemokines (e.g., CCL7), and matrix metalloproteinases (MMPs). TNF-α increased myogenic factor 5 (Myf5) but decreased MyoD protein levels and stimulated the release of MMP-9, MMP-10, and MMP-13. TNF-α also upregulates versican and myostatin mRNA. Overall, our study demonstrates the TNF-α modulation of distinct gene expression patterns and signaling pathways that likely contribute to enhanced myoblast proliferation while suppressing premature differentiation after muscle injury. Elucidating the mechanisms involved in skeletal muscle regeneration can aid in the development of regeneration-enhancing therapeutics.
Keywords: cytokine; inflammation; muscle regeneration.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
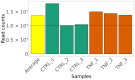
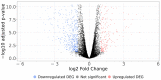
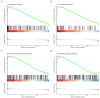
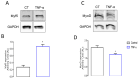
References
Publication types
MeSH terms
Substances
Grants and funding
- 2015/25437-8/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2015/50040-4 and 2020/13139-0/Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo (FAPESP)/ GlaxoSmithKline
- 2018/10937-3/Fundação de Amparo à Pesquisa do Estado de São Paulo
- RVO 68378050 and LM2023036/Czech Centre for Phenogenomics provided by the Ministry of Education, Youth and Sports of the Czech Republic
LinkOut - more resources
Full Text Sources
Miscellaneous

