Safety of rezafungin as a long-term treatment option in two patients with complicated fungal infections: two cases from Lecco Hospital (Italy)
- PMID: 38995032
- PMCID: PMC11304680
- DOI: 10.1128/aac.00750-24
Safety of rezafungin as a long-term treatment option in two patients with complicated fungal infections: two cases from Lecco Hospital (Italy)
Abstract
Rezafungin is an echinocandin characterized by a long elimination half-life which allows for weekly administration. It has been recently approved for the treatment of candidemia. Few data are available about the long-term use of rezafungin and its use for deep infections like endocarditis and osteomyelitis. We describe our experience with its prolonged use in two azole-resistant Candida infections: a case of sacral osteomyelitis and a prosthetic valve endocarditis also involving a thoracic endovascular aneurysm repair.
Keywords: Candida; antifungal resistance; rezafungin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

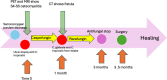

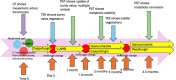
Comment in
-
Case Commentary: Extending our therapeutic range against multidrug-resistant Candida.Antimicrob Agents Chemother. 2024 Aug 7;68(8):e0084724. doi: 10.1128/aac.00847-24. Epub 2024 Jul 22. Antimicrob Agents Chemother. 2024. PMID: 39037274 Free PMC article.
References
-
- Thompson GR, Soriano A, Skoutelis A, Vazquez JA, Honore PM, Horcajada JP, Spapen H, Bassetti M, Ostrosky-Zeichner L, Das AF, Viani RM, Sandison T, Pappas PG. 2021. Rezafungin versus caspofungin in a phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis: the STRIVE trial. Clin Infect Dis 73:e3647–e3655. doi: 10.1093/cid/ciaa1380 - DOI - PMC - PubMed
-
- Thompson GR, Soriano A, Cornely OA, Kullberg BJ, Kollef M, Vazquez J, Honore PM, Bassetti M, Pullman J, Chayakulkeeree M, et al. 2023. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): a multicentre, double-blind, double-dummy, randomised phase 3 trial. The Lancet 401:49–59. doi: 10.1016/S0140-6736(22)02324-8 - DOI - PubMed
-
- Locke JB, Pillar CM, Castanheira M, Carvalhaes CG, Andes D, Aram JA, Andrzejewski C, Bartizal K, Das AF, Sandison T, Thompson GR, Pappas PG. 2024. Outcomes by Candida spp. in the ReSTORE phase 3 trial of rezafungin versus caspofungin for candidemia and/or invasive candidiasis. Antimicrob Agents Chemother 68:e0158423. doi: 10.1128/aac.01584-23 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

