Evolutionary trajectory for nuclear functions of ciliary transport complex proteins
- PMID: 38995044
- PMCID: PMC11426024
- DOI: 10.1128/mmbr.00006-24
Evolutionary trajectory for nuclear functions of ciliary transport complex proteins
Abstract
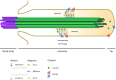
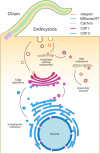
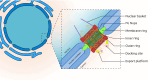
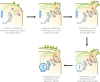
SUMMARYCilia and the nucleus were two defining features of the last eukaryotic common ancestor. In early eukaryotic evolution, these structures evolved through the diversification of a common membrane-coating ancestor, the protocoatomer. While in cilia, the descendants of this protein complex evolved into parts of the intraflagellar transport complexes and BBSome, the nucleus gained its selectivity by recruiting protocoatomer-like proteins to the nuclear envelope to form the selective nuclear pore complexes. Recent studies show a growing number of proteins shared between the proteomes of the respective organelles, and it is currently unknown how ciliary transport proteins could acquire nuclear functions and vice versa. The nuclear functions of ciliary proteins are still observable today and remain relevant for the understanding of the disease mechanisms behind ciliopathies. In this work, we review the evolutionary history of cilia and nucleus and their respective defining proteins and integrate current knowledge into theories for early eukaryotic evolution. We postulate a scenario where both compartments co-evolved and that fits current models of eukaryotic evolution, explaining how ciliary proteins and nucleoporins acquired their dual functions.
Keywords: cell biology; cilia; eukaryogenesis; eukaryotes; evolution; intraflagellar transport; last eukaryotic common ancestor; molecular biology; nuclear pore complex; nucleus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

