Unveiling the stochastic nature of human heteropolymer ferritin self-assembly mechanism
- PMID: 38995055
- PMCID: PMC11241160
- DOI: 10.1002/pro.5104
Unveiling the stochastic nature of human heteropolymer ferritin self-assembly mechanism
Abstract
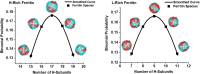
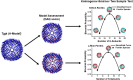
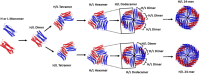
Despite ferritin's critical role in regulating cellular and systemic iron levels, our understanding of the structure and assembly mechanism of isoferritins, discovered over eight decades ago, remains limited. Unveiling how the composition and molecular architecture of hetero-oligomeric ferritins confer distinct functionality to isoferritins is essential to understanding how the structural intricacies of H and L subunits influence their interactions with cellular machinery. In this study, ferritin heteropolymers with specific H to L subunit ratios were synthesized using a uniquely engineered plasmid design, followed by high-resolution cryo-electron microscopy analysis and deep learning-based amino acid modeling. Our structural examination revealed unique architectural features during the self-assembly mechanism of heteropolymer ferritins and demonstrated a significant preference for H-L heterodimer formation over H-H or L-L homodimers. Unexpectedly, while dimers seem essential building blocks in the protein self-assembly process, the overall mechanism of ferritin self-assembly is observed to proceed randomly through diverse pathways. The physiological significance of these findings is discussed including how ferritin microheterogeneity could represent a tissue-specific adaptation process that imparts distinctive tissue-specific functions to isoferritins.
Keywords: cryo‐EM; ferritin microheterogeneity; ferritin subunits; human heteropolymer ferritin; isoferritins; self‐assembly mechanism.
© 2024 The Protein Society.
Figures






Similar articles
-
Challenges in Exploiting Human H Ferritin Nanoparticles for Drug Delivery: Navigating Physiological Constraints.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024 Nov-Dec;16(6):e2016. doi: 10.1002/wnan.2016. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024. PMID: 39541599 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Adapting Safety Plans for Autistic Adults with Involvement from the Autism Community.Autism Adulthood. 2025 May 28;7(3):293-302. doi: 10.1089/aut.2023.0124. eCollection 2025 Jun. Autism Adulthood. 2025. PMID: 40539213
-
"In a State of Flow": A Qualitative Examination of Autistic Adults' Phenomenological Experiences of Task Immersion.Autism Adulthood. 2024 Sep 16;6(3):362-373. doi: 10.1089/aut.2023.0032. eCollection 2024 Sep. Autism Adulthood. 2024. PMID: 39371355
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
Cited by
-
Rational pore engineering reveals the relative contribution of enzymatic sites and self-assembly towards rapid ferroxidase activity and mineralization: impact of electrostatic guiding and cage-confinement in bacterioferritin.Chem Sci. 2025 Jan 20;16(9):3978-3997. doi: 10.1039/d4sc07021f. eCollection 2025 Feb 26. Chem Sci. 2025. PMID: 39886445 Free PMC article.
-
Controlling nanocage assembly, towards developing a one-health "plug & play" platform for targeted therapy.Chem Commun (Camb). 2025 Aug 18. doi: 10.1039/d5cc03592a. Online ahead of print. Chem Commun (Camb). 2025. PMID: 40824119 Free PMC article. Review.
-
A Brief History of Ferritin, an Ancient and Versatile Protein.Int J Mol Sci. 2024 Dec 29;26(1):206. doi: 10.3390/ijms26010206. Int J Mol Sci. 2024. PMID: 39796064 Free PMC article. Review.
-
AI-based quality assessment methods for protein structure models from cryo-EM.Curr Res Struct Biol. 2025 Feb 2;9:100164. doi: 10.1016/j.crstbi.2025.100164. eCollection 2025 Jun. Curr Res Struct Biol. 2025. PMID: 39996138 Free PMC article. Review.
References
-
- Andrews SC, Smith JM, Hawkins C, Williams JM, Harrison PM, Guest JR. Overproduction, purification, and characterization of the bacterioferritin of Escherichia coli and a C‐terminally extended variant. Eur J Biochem. 1993;213:329–338. - PubMed
-
- Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta, Gen Subj. 2009;1790(7):589–599. - PubMed
-
- Ayoub M, Levoye A, Delagrange P, Jockers R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol Pharmacol. 2004;66(2):312–321. - PubMed
-
- Beck M, Covino R, Hänelt I, Müller‐McNicoll M. Understanding the cell: future views of structural biology. Cell. 2024;187(3):545–562. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

