CSF1R Inhibition in Patients with Advanced Solid Tumors or Tenosynovial Giant Cell Tumor: A Phase I Study of Vimseltinib
- PMID: 38995311
- PMCID: PMC11393540
- DOI: 10.1158/1078-0432.CCR-24-0103
CSF1R Inhibition in Patients with Advanced Solid Tumors or Tenosynovial Giant Cell Tumor: A Phase I Study of Vimseltinib
Abstract
Purpose: Tenosynovial giant cell tumor (TGCT) is a locally aggressive neoplasm caused by dysregulation of the colony-stimulating factor 1 (CSF1) gene and overexpression of the CSF1 ligand. Surgery is the standard of care for most patients, but there are limited treatment options for patients with TGCT not amenable to surgery. This study evaluates vimseltinib, an investigational, oral, switch-control tyrosine kinase inhibitor designed to selectively and potently inhibit the CSF1 receptor.
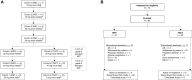
Patients and methods: This first-in-human, multicenter, open-label phase I/II study of vimseltinib in patients with malignant solid tumors (N = 37) or TGCT not amenable to surgery (N = 32) followed a pharmacologically guided 3 + 3 study design (NCT03069469). The primary objectives were to assess safety and tolerability, determine the recommended phase II dose, and characterize the pharmacokinetics; exploratory objectives included pharmacodynamics and efficacy.
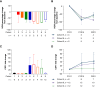
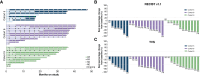
Results: Vimseltinib was well tolerated; the majority of non-laboratory treatment-emergent adverse events were of grade 1/2 severity. There was no evidence of cholestatic hepatotoxicity or drug-induced liver injury. The recommended phase II dose was determined to be 30 mg twice weekly (no loading dose), and vimseltinib plasma exposure increased with the dose. In patients with TGCT, the median treatment duration was 25.1 months (range, 0.7-46.9), and the objective response rate as assessed by independent radiological review using RECIST version 1.1 was 72%.
Conclusions: Vimseltinib demonstrated long-term tolerability, manageable safety, dose-dependent exposure, and robust antitumor activity in patients with TGCT not amenable to surgery.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A.A. Razak reports grants from Deciphera during the conduct of the study, as well as personal fees from Medison outside the submitted work. M.H. Taylor reports other support from Exelixis, Bristol Myers Squibb, OncoSec, Blueprint Medicines, Eisai, Inc., Merck, Immune-Onc, Pfizer, Regeneron, and Sanofi/Genzyme outside the submitted work. T.M. Bauer reports grants from Deciphera during the conduct of the study, as well as personal fees from Pfizer, Bayer, Eli Lilly and Company, Sanofi, and AVEO outside the submitted work. B. Wilky reports personal fees from Deciphera during the conduct of the study, as well as personal fees from SpringWorks and Boehringer Ingelheim outside the submitted work. J. Martín-Broto reports grants from Deciphera during the conduct of the study, as well as grants from Bristol Myers Squibb, Eisai, Immix Biopharma, Pfizer, AROG, Lixte, Karyopharm, Celgene, Blueprint, Adaptimmune, Daichii Sankyo, Rain Therapeutics, Inhibrx, Ayala Pharmaceuticals, Philogen, Cebiotex, PTC Therapeutics, Inc., and SpringWorks Therapeutics outside the submitted work; grants and personal fees from PharmaMar, Eli Lilly and Company, Bayer, GSK, and Amgen; and personal fees from Boehringer Ingelheim, Novartis, Roche, Tecnofarma, and Asofarma. A.F. Gonzalez reports personal fees from Roche, Novartis, AstraZeneca, MSD, Pfizer, Eli Lilly and Company, and Esteve as well as personal fees and nonfinancial support from Gilead and Daiichi Sankyo outside the submitted work. P. Rutkowski reports personal fees from MSD, Bristol Myers Squibb, Pierre Fabre, Deciphera, Genesis Pharma, Novartis, and Medison Pharma outside the submitted work. T. Alcindor reports personal fees from Astellas, Bayer, AstraZeneca, Bristol Myers Squibb, and Merck and other support from Epizyme, SpringWorks Therapeutics, Deciphera, and EMD Serono during the conduct of the study. S. Stacchiotti reports grants from Deciphera during the conduct of the study, as well as grants from Abbisko, Novartis, SpringWorks, Advenchen, Epizyme, Foghorn, Hutchinson, GSK, and Inhibrx; grants and personal fees from Deciphera, Daiichi, Boehringer, and PharmaMar; and personal fees from Bayer, Gentili, Ikena, Ipsen, Servier, Regeneron, PharmaEssentia, Agenus, and Nec Oncology outside the submitted work. M. van de Sande reports other support from Deciphera during the conduct of the study. A.J. Wagner reports grants and personal fees from Deciphera during the conduct of the study, as well as grants and personal fees from Boehringer Ingelheim, Cogent Biosciences, and Daiichi Sankyo and personal fees from Eli Lilly and Company, BioAtla, Servier, Inhibrx, Kymera, and PharmaEssentia outside the submitted work. N. Bernthal reports personal fees from Deciphera and Daiichi Sankyo outside the submitted work. L.E. Davis reports personal fees from SpringWorks, Daiichi Sankyo, Inhibrx, and Regeneron outside the submitted work. J. Vuky reports other support from Deciphera during the conduct of the study, as well as other support from Deciphera, Arvinas, Bristol Myers Squibb, Fortis, Exelixis, Astellas, Eli Lilly and Company, AstraZeneca, Merck, and Pfizer outside the submitted work; J. Vuky also reports current employment with Genentech but not during the conduct of the study. C. Tait reports employment with Deciphera and ownership of stock in Deciphera. B. Matin reports employment with Deciphera Pharmaceuticals, LLC, and holding stock/other ownership interests in Deciphera Pharmaceuticals, LLC. S. Narasimhan reports personal fees and other support from Deciphera Pharmaceuticals and other support from Red Nucleus during the conduct of the study, as well as personal fees and other support from Deciphera Pharmaceuticals outside the submitted work. M.G. Sharma reports personal fees and other support from Deciphera Pharmaceuticals during the conduct of the study. R. Ruiz-Soto reports other support from Deciphera Pharmaceuticals during the conduct of the study and outside the submitted work; R. Ruiz-Soto also reports a patent for Methods of Treating Disorders Using CSF1R Inhibitors issued and pending. M.L. Sherman reports personal fees, nonfinancial support, and other support from Deciphera Pharmaceuticals during the conduct of the study, as well as personal fees, nonfinancial support, and other support from Pieris Pharmaceuticals outside the submitted work. W.D. Tap reports other support from Deciphera during the conduct of the study; personal fees from Avacta, Inhibrx, Aadi, Abbisko, Ikena, Curadev, Servier, Deciphera, Eli Lilly and Company, Foghorn, Pharma Essential, Daiichi, AmMax Bio, Cogent, BioAtla, Boehringer, Ipsen, Bayer, and C4 Therapeutics outside the submitted work; a patent for Enigma CDK4 Inhibition issued; and relationships with Atropos, Certis (founder stock ownership and scientific advisory board), Innova (stock ownership and scientific advisory board), and Osteosarcoma Institute (scientific advisory board). No disclosures were reported by the other authors.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

