Immunogenicity, Safety, and Efficacy of a Tetravalent Dengue Vaccine in Children and Adolescents: An Analysis by Age Group
- PMID: 38995684
- PMCID: PMC11797386
- DOI: 10.1093/cid/ciae369
Immunogenicity, Safety, and Efficacy of a Tetravalent Dengue Vaccine in Children and Adolescents: An Analysis by Age Group
Erratum in
-
Correction to: Immunogenicity, Safety, and Efficacy of a Tetravalent Dengue Vaccine in Children and Adolescents: An Analysis by Age Group.Clin Infect Dis. 2024 Dec 17;79(6):1543. doi: 10.1093/cid/ciae504. Clin Infect Dis. 2024. PMID: 39545821 Free PMC article. No abstract available.
Abstract
Background: Dengue is an increasing threat to global health. This exploratory analysis evaluated the immunogenicity, safety, and vaccine efficacy (VE) of a live-attenuated tetravalent dengue vaccine (TAK-003) in participants enrolled in the phase 3 DEN-301 trial (NCT02747927), stratified by baseline age (4-5 years, 6-11 years, or 12-16 years).
Methods: Participants were randomized 2:1 to receive 2 doses of TAK-003, administered 3 months apart, or placebo. Dengue serostatus was evaluated at enrolment. VE against virologically confirmed dengue (VCD) and hospitalized VCD; immunogenicity (geometric mean titers [GMTs]); and safety were evaluated per age group through ∼4 years postvaccination.
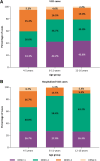
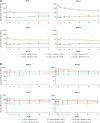
Results: VE against VCD across serotypes was 43.5% (95% confidence interval [CI]: 25.3%, 57.3%) for 4-5 year-olds; 63.5% (95% CI: 56.9%, 69.1%) for 6-11 year-olds, and 67.7% (95% CI: 57.8%, 75.2%) for 12-16 year-olds. VE against hospitalized VCD was 63.8% (95% CI: 21.1%, 83.4%), 85.1% (95% CI: 77.1%, 90.3%), and 89.7% (95% CI: 77.9%, 95.2%), for the 3 age groups, respectively. GMTs remained elevated against all 4 serotypes for ∼4 years postvaccination, with no evident differences across age groups. No clear differences in safety by age were identified.
Conclusions: This exploratory analysis shows TAK-003 was efficacious in dengue prevention across age groups in children and adolescents 4-16 years of age living in dengue endemic areas. Relatively lower VE in 4-5 year-olds was potentially confounded by causative serotype distribution, small sample size, and VE by serotype, and should be considered in benefit-risk evaluations in this age group.
Keywords: child; dengue vaccine; efficacy; immunogenicity; safety.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Figures

References
-
- World Health Organization . Dengue and severe dengue. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed 13 June 2024.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

