Thermal Truncation of Heptamethine Cyanine Dyes
- PMID: 38995720
- PMCID: PMC11273355
- DOI: 10.1021/jacs.4c02116
Thermal Truncation of Heptamethine Cyanine Dyes
Abstract
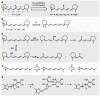
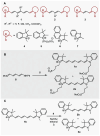
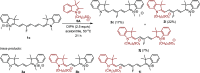
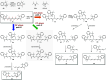
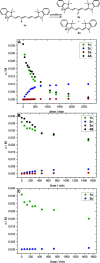
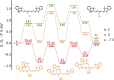
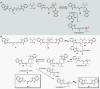
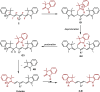
Cyanine dyes are a class of organic, usually cationic molecules containing two nitrogen centers linked through conjugated polymethine chains. The synthesis and reactivity of cyanine derivatives have been extensively investigated for decades. Unlike the recently described phototruncation process, the thermal truncation (chain shortening) reaction is a phenomenon that has rarely been reported for these important fluorophores. Here, we present a systematic investigation of the truncation of heptamethine cyanines (Cy7) to pentamethine (Cy5) and trimethine (Cy3) cyanines via homogeneous, acid-base-catalyzed nucleophilic exchange reactions. We demonstrate how different substituents at the C3' and C4' positions of the chain and different heterocyclic end groups, the presence of bases, nucleophiles, and oxygen, solvent properties, and temperature affect the truncation process. The mechanism of chain shortening, studied by various analytical and spectroscopic techniques, was verified by extensive ab initio calculation, implying the necessity to model catalytic reactions by highly correlated wave function-based methods. In this study, we provide critical insight into the reactivity of cyanine polyene chains and elucidate the truncation mechanism and methods to mitigate side processes that can occur during the synthesis of cyanine derivatives. In addition, we offer alternative routes to the preparation of symmetrical and unsymmetrical meso-substituted Cy5 derivatives.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Choi H. S.; Nasr K.; Alyabyev S.; Feith D.; Lee J. H.; Kim S. H.; Ashitate Y.; Hyun H.; Patonay G.; Strekowski L.; Henary M.; Frangioni J. V. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew. Chem., Int. Ed. 2011, 50, 6258–6263. 10.1002/anie.201102459. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

