Smartphone-Based Assessment of Mobility and Manual Dexterity in Adult People with Spinal Muscular Atrophy
- PMID: 38995798
- PMCID: PMC11380318
- DOI: 10.3233/JND-240004
Smartphone-Based Assessment of Mobility and Manual Dexterity in Adult People with Spinal Muscular Atrophy
Abstract
Background: More responsive, reliable, and clinically valid endpoints of disability are essential to reduce size, duration, and burden of clinical trials in adult persons with spinal muscular atrophy (aPwSMA).
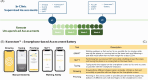
Objective: The aim is to investigate the feasibility of smartphone-based assessments in aPwSMA and provide evidence on the reliability and construct validity of sensor-derived measures (SDMs) of mobility and manual dexterity collected remotely in aPwSMA.
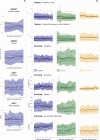
Methods: Data were collected from 59 aPwSMA (23 walkers, 20 sitters and 16 non-sitters) and 30 age-matched healthy controls (HC). SDMs were extracted from five smartphone-based tests capturing mobility and manual dexterity, which were administered in-clinic and remotely in daily life for four weeks. Reliability (Intraclass Correlation Coefficients, ICC) and construct validity (ability to discriminate between HC and aPwSMA and correlations with Revised Upper Limb Module, RULM and Hammersmith Functional Scale - Expanded HFMSE) were quantified for all SDMs.
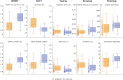
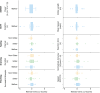
Results: The smartphone-based assessments proved feasible, with 92.1% average adherence in aPwSMA. The SDMs allowed to reliably assess both mobility and dexterity (ICC > 0.75 for 14/22 SDMs). Twenty-one out of 22 SDMs significantly discriminated between HC and aPwSMA. The highest correlations with the RULM were observed for SDMs from the manual dexterity tests in both non-sitters (Typing, ρ= 0.78) and sitters (Pinching, ρ= 0.75). In walkers, the highest correlation was between mobility tests and HFMSE (5 U-Turns, ρ= 0.79).
Conclusions: This exploratory study provides preliminary evidence for the usability of smartphone-based assessments of mobility and manual dexterity in aPwSMA when deployed remotely in participants' daily life. Reliability and construct validity of SDMs remotely collected in real-life was demonstrated, which is a pre-requisite for their use in longitudinal trials. Additionally, three novel smartphone-based performance outcome assessments were successfully established for aPwSMA. Upon further validation of responsiveness to interventions, this technology holds potential to increase the efficiency of clinical trials in aPwSMA.
Keywords: Remote monitoring; accelerometers; drawing; pinching; turning; typing; walking; wearable sensors.
Conflict of interest statement
EAB, GC, CK, AK, CM, JPA, CZ, CS, KME, and SB were Biogen employees at the time of writing and might hold stock of the Company. HSL has received advisory fees from Biogen outside of the submitted work. SW has received research grant by the DGM— Deutsche Gesellschaft für Muskelkranke e.V. He has served on advisory boards for Alexion Pharma, UCB Pharma GmbH, AMICUS Therapeutics GmbH, and Sanofi Genzyme GmbH. He received funding for travel or speaker Honoraria from Sanofi-Aventis Germany GmbH; SH Glykogenose Gesellschaft; AbbVie Germany GmbH; Recordati Pharma GmbH; CSLBehring GmbH; Alexion Pharma GmbH; Desitin Germany; Akcea GmbH. SP received honoraria as speaker/consultant from Biogen GmbH, Roche, Novartis, Teva, Cytokinetics Inc., Desitin, Italfarmaco, Amylyx, and Zambon; and grants from DGM e.V, Federal Ministry of Education and Research, German Israeli Foundation for Scientific Research and Development, EU Joint Program for Neurodegenerative Disease Research and Neurodegenerative Research, Inc., outside of the submitted work. MW has received advisory board and consultant honoraria from Biogen and Hoffmann-La Roche, speaker honoraria and travel support for conference attendance from Biogen, outside of the submitted work, and is a member of the
European Reference Network for Neuromuscular Diseases (ERN EURO-NMD). WMC attended advisory boards of AveXis Pharma, Biogen Pharma GmbH, Novartis Pharma GmbH, Pfizer Inc., PharNEXT, PTC Therapeutics, Roche Pharma AG, RTI HS, Santhera Pharmaceuticals, Sarepta Therapeutics Inc, received funding for travel and speaker honoraria from Biogen Pharma GmbH, Colloquium Neurologicum, Novartis Pharma GmbH, PTC Therapeutics, Santhera Pharmaceuticals, and consulted for Affinia, Audentes Therapeutics, Avexis, Biogen Pharma GmbH, BridgeBio, Edgewise, Fulcrum Therapeutics, Grünenthal PharmaML Bio, Novartis Pharma GmbH, Pfizer Inc., PharNEXT, PTC therapeutics, Roche Pharma AG. RG has received personal fees from Biogen, Hofmann-La Roche, Zambon and ITF Pharma and research support from Biogen, all support was received outside the scope of the submitted work. TH received compensation for adboard consultancy, speaker fees and research support from Biogen and Roche and research support from Novartis.
Figures

References
-
- Hagenacker T, Wurster CD, Günther R, Schreiber-Katz O, Osmanovic A, Petri S, Weiler M, Ziegler A, Kuttler J, Koch JC, Schneider I, Wunderlich G, Schloss N, Lehmann HC, Cordts I, Deschauer M, Lingor P, Kamm C, Stolte B, Pietruck L, Totzeck A, Kizina K, Mönninghoff C, von Velsen O, Ose C, Reichmann H, Forsting M, Pechmann A, Kirschner J, Ludolph AC, Hermann A, Kleinschnitz C. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020;19(4):317–25. - PubMed
-
- Wirth B, Karakaya M, Kye MJ, Mendoza-Ferreira N. Twenty-Five Years of Spinal Muscular Atrophy Research: From Phenotype to Genotype to Therapy, and What Comes Next. Annu Rev Genomics Hum Genet. 2020;21, 231–61. - PubMed
-
- Coratti G, Messina S, Lucibello S, Pera MC, Montes J, Pasternak A, Bovis F, Exposito Escudero J, Mazzone ES, Mayhew A, Glanzman AM, Young SD, Salazar R, Duong T, Muni Lofra R, De Sanctis R, Carnicella S, Milev E, Civitello M, Pane M, Scoto M, Bettolo CM, Antonaci L, Frongia A, Sframeli M, Vita GL, D’Amico A, Van Den Hauwe M, Albamonte E, Goemans N, Darras BT, Bertini E, Sansone V, Day J, Nascimento Osorio A, Bruno C, Muntoni F, De Vivo DC, Finkel RS, Mercuri E. Clinical Variability in Spinal Muscular Atrophy Type III. Ann Neurol. 2020;88(6):1109–17. - PubMed
-
- Maggi L, Bello L, Bonanno S, Govoni A, Caponnetto C, Passamano L, Grandis M, Trojsi F, Cerri F, Ferraro M, Bozzoni V, Caumo L, Piras R, Tanel R, Saccani E, Meneri M, Vacchiano V, Ricci G, Soraru’ G, D’Errico E, Tramacere I, Bortolani S, Pavesi G, Zanin R, Silvestrini M, Politano L, Schenone A, Previtali SC, Berardinelli A, Turri M, Verriello L, Coccia M, Mantegazza R, Liguori R, Filosto M, Marrosu G, Siciliano G, Simone IL, Mongini T, Comi G, Pegoraro E. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry. 2020;91(11):1166–74. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

