Injectable shear-thinning hydrogels promote oligodendrocyte progenitor cell survival and remyelination in the central nervous system
- PMID: 38996029
- PMCID: PMC11244542
- DOI: 10.1126/sciadv.adk9918
Injectable shear-thinning hydrogels promote oligodendrocyte progenitor cell survival and remyelination in the central nervous system
Abstract
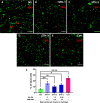
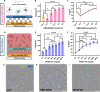
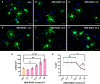
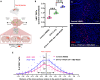
Cell therapy for the treatment of demyelinating diseases such as multiple sclerosis is hampered by poor survival of donor oligodendrocyte cell preparations, resulting in limited therapeutic outcomes. Excessive cell death leads to the release of intracellular alloantigens, which likely exacerbate local inflammation and may predispose the graft to eventual rejection. Here, we engineered innovative cell-instructive shear-thinning hydrogels (STHs) with tunable viscoelasticity and bioactivity for minimally invasive delivery of primary human oligodendrocyte progenitor cells (hOPCs) to the brain of a shiverer/rag2 mouse, a model of congenital hypomyelinating disease. The STHs enabled immobilization of prosurvival signals, including a recombinantly designed bidomain peptide and platelet-derived growth factor. Notably, STHs reduced the death rate of hOPCs significantly, promoted the production of myelinating oligodendrocytes, and enhanced myelination of the mouse brain 12 weeks post-implantation. Our results demonstrate the potential of STHs loaded with biological cues to improve cell therapies for the treatment of devastating myelopathies.
Figures


Similar articles
-
C1ql1 expression in oligodendrocyte progenitor cells promotes oligodendrocyte differentiation.FEBS J. 2025 Jan;292(1):52-74. doi: 10.1111/febs.17256. Epub 2024 Sep 11. FEBS J. 2025. PMID: 39257292
-
ACT-1004-1239, a first-in-class CXCR7 antagonist with both immunomodulatory and promyelinating effects for the treatment of inflammatory demyelinating diseases.FASEB J. 2021 Mar;35(3):e21431. doi: 10.1096/fj.202002465R. FASEB J. 2021. PMID: 33595155 Free PMC article.
-
Trametinib, an anti-tumor drug, promotes oligodendrocytes generation and myelin formation.Acta Pharmacol Sin. 2024 Dec;45(12):2527-2539. doi: 10.1038/s41401-024-01313-9. Epub 2024 Jun 13. Acta Pharmacol Sin. 2024. PMID: 38871922
-
Spinal Cord Injury Remyelination: Pathways to Therapies.Int J Mol Sci. 2025 Jul 26;26(15):7249. doi: 10.3390/ijms26157249. Int J Mol Sci. 2025. PMID: 40806380 Free PMC article. Review.
-
Remyelination in the Central Nervous System.Cold Spring Harb Perspect Biol. 2024 Mar 1;16(3):a041371. doi: 10.1101/cshperspect.a041371. Cold Spring Harb Perspect Biol. 2024. PMID: 38316552 Review.
Cited by
-
Injectable nanocomposite hydrogel for localized precision delivery of dexamethasone after traumatic brain injury: dual modulation of neuroinflammation and blood-brain barrier restoration.J Transl Med. 2025 May 23;23(1):579. doi: 10.1186/s12967-025-06528-w. J Transl Med. 2025. PMID: 40410771 Free PMC article.
-
Multifunctional injectable hydrogel incorporating EGCG-Cu complexes for synergistic antibacterial, immunomodulatory, and osteogenic therapy in periodontitis.Mater Today Bio. 2025 May 28;32:101907. doi: 10.1016/j.mtbio.2025.101907. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40520560 Free PMC article.
References
-
- Tepavčević V., Lubetzki C., Oligodendrocyte progenitor cell recruitment and remyelination in multiple sclerosis: The more, the merrier? Brain 145, 4178–4192 (2022). - PubMed
-
- Windrem M. S., Schanz S. J., Guo M., Tian G. F., Washco V., Stanwood N., Rasband M., Roy N. S., Nedergaard M., Havton L. A., Wang S., Goldman S. A., Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell 2, 553–565 (2008). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

