Comprehensive Review on the Use of Oral Cholera Vaccine (OCV) in Ethiopia: 2019 to 2023
- PMID: 38996040
- PMCID: PMC11244176
- DOI: 10.1093/cid/ciae194
Comprehensive Review on the Use of Oral Cholera Vaccine (OCV) in Ethiopia: 2019 to 2023
Abstract
Background: Cholera outbreaks in Ethiopia necessitate frequent mass oral cholera vaccine (OCV) campaigns. Despite this, there is a notable absence of a comprehensive summary of these campaigns. Understanding national OCV vaccination history is essential to design appropriate and effective cholera control strategies. Here, we aimed to retrospectively review all OCV vaccination campaigns conducted across Ethiopia between 2019 and 2023.
Methods: The OCV request records from 2019 to October 2023 and vaccination campaign reports for the period from 2019 to December 2023 were retrospectively accessed from the Ethiopia Public Health Institute (EPHI) database. Descriptive analysis was conducted using the retrospective data collected.
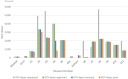
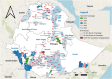
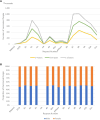
Results: From 2019 to October 2023, Ethiopian government requested 32 044 576 OCV doses (31 899 576 doses to global stockpile; 145 000 doses to outside of stockpile). Around 66.3% of requested doses were approved; of which 90.4% were received. Fifteen OCV campaigns (12 reactive and 3 pre-emptive) were conducted, including five two-dose campaigns with varying dose intervals and single-dose campaigns partially in 2019 and entirely in 2021, 2022 and 2023. Overall vaccine administrative coverage was high; except for Tigray region (41.8% in the 1st round; 2nd round didn't occur). The vaccine administrative coverage records were documented, but no OCV coverage survey data was available.
Conclusions: This study represents the first comprehensive review of OCV campaigns in Ethiopia spanning the last five years. Its findings offer valuable insights into informing future cholera control strategies, underscoring the importance of monitoring and evaluation despite resource constraints. Addressing the limitations in coverage survey data availability is crucial for enhancing the efficacy of future campaigns.
Keywords: Cholera; Ethiopia; OCV; OCV dosing schedules; global OCV stockpile.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- World Health Organization . Cholera vaccine. Available at: https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases.... Accessed 15 December 2023.
-
- Global Task Force on Cholera Control . Ending cholera a global roadmap to 2030. Available at: https://www.gtfcc.org/wp-content/uploads/2019/10/gtfcc-ending-cholera-a-.... Accessed 11 December 2023.
-
- GTFCC OCV Working Group Annual Meeting . Gavi support for OCV programme. Available at: https://www.gtfcc.org/wp-content/uploads/2022/08/9th-meeting-of-gtfcc-wg.... Accessed 11 December 2023.
-
- Global Task Force on Cholera Control . GTFCC OCV dashboard. Available at: https://apps.epicentre-msf.org/public/app/gtfcc. Accessed 10 December 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

