Effectiveness and Safety of an Emergency Department Code Sepsis Protocol: A Pragmatic Clinical Trial
- PMID: 38996086
- PMCID: PMC11568504
- DOI: 10.1513/AnnalsATS.202403-286OC
Effectiveness and Safety of an Emergency Department Code Sepsis Protocol: A Pragmatic Clinical Trial
Abstract
Rationale: Sepsis care delivery-including the initiation of prompt, appropriate antimicrobials-remains suboptimal. Objectives: This study was conducted to determine direct and off-target effects of emergency department (ED) sepsis care reorganization. Methods: This pragmatic pilot trial enrolled adult patients who presented from November 2019 to February 2021 to an ED in Utah before and after implementation of a multimodal, team-based "Code Sepsis" protocol. Patients who presented to two other EDs where usual care was continued served as contemporaneous control subjects. The primary outcome was door-to-antimicrobial time among patients meeting Sepsis-3 criteria before ED departure. Secondary and safety outcomes included all-cause 30-day mortality, antimicrobial utilization and overtreatment, and antimicrobial-associated adverse events. Multivariable regression analyses used difference-in-differences methods to account for trends in outcomes unrelated to the studied intervention. Results: Code Sepsis protocol activation (N = 307) exhibited 8.5% sensitivity and 66% positive predictive value for patients meeting sepsis criteria before ED departure. Among 10,151 patients who met sepsis criteria during the study, adjusted difference-in-differences analysis demonstrated a 13-minute (95% confidence interval = 7-19) decrease in door-to-antimicrobial time associated with Code Sepsis implementation (P < 0.001). Mortality and clinical safety outcomes were unchanged, but Code Sepsis implementation was associated with increased false-positive presumptive infection diagnoses among patients who met sepsis criteria in the ED and increased antimicrobial utilization. Conclusions: Implementation of a team-based protocol for rapid sepsis evaluation and treatment during the coronavirus disease (COVID-19) pandemic's first year was associated with decreased ED door-to-antimicrobial time but also increased antimicrobial utilization. Measurement of both patient-centered and off-target effects of sepsis care improvement interventions is essential to comprehensive assessment of their value. Clinical trial registered with www.clinicaltrials.gov (NCT04148989).
Keywords: antibiotic time; emergency medicine; health services; sepsis.
Figures
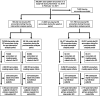
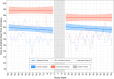

References
-
- Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med . 2021;49:e1063–e1143. - PubMed
-
- Corl KA, Zeba F, Caffrey AR, Hermenau M, Lopes V, Phillips G, et al. Delay in antibiotic administration is associated with mortality among septic shock patients with Staphylococcus aureus bacteremia. Crit Care Med . 2020;48:525–532. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

