Immune mechanisms in fibrotic interstitial lung disease
- PMID: 38996486
- PMCID: PMC11246539
- DOI: 10.1016/j.cell.2024.05.015
Immune mechanisms in fibrotic interstitial lung disease
Abstract
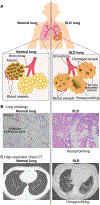
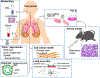
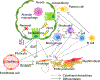
Fibrotic interstitial lung diseases (fILDs) have poor survival rates and lack effective therapies. Despite evidence for immune mechanisms in lung fibrosis, immunotherapies have been unsuccessful for major types of fILD. Here, we review immunological mechanisms in lung fibrosis that have the potential to impact clinical practice. We first examine innate immunity, which is broadly involved across fILD subtypes. We illustrate how innate immunity in fILD involves a complex interplay of multiple cell subpopulations and molecular pathways. We then review the growing evidence for adaptive immunity in lung fibrosis to provoke a re-examination of its role in clinical fILD. We close with future directions to address key knowledge gaps in fILD pathobiology: (1) longitudinal studies emphasizing early-stage clinical disease, (2) immune mechanisms of acute exacerbations, and (3) next-generation immunophenotyping integrating spatial, genetic, and single-cell approaches. Advances in these areas are essential for the future of precision medicine and immunotherapy in fILD.
Keywords: fibroblasts; idiopathic pulmonary fibrosis; immune system; interstitial lung diseases; pulmonary fibrosis.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests In disclosures unrelated to this work: M.K. received research funding from GlaxoSmithKline. T.J.D. received research support from Bayer and Bristol Myers Squibb, consulting fees from Boehringer Ingelheim and L.E.K. consulting, and has been part of a clinical trial funded by Genentech, all unrelated to this study. L.K.D. received research grants from the Brazilian Ministry of Health (PROADI-SUS), Boehringer Ingelheim, and Bristol-Myers-Squibb. J.S.L. received grants from the NIH and Boehringer Ingelheim, an unrestricted research gift from Pliant, and consulting fees from Blade, Boehringer Ingelheim, United Therapeutics, Astra Zenca, and Eleven P15, all outside the submitted work. J.S.L. serves on the DSMB for UT, Avalyn and is an advisor for the Pulmonary Fibrosis Foundation, all outside the submitted work. L.K.D. received consulting fees from Boehringer Ingelheim, Roche, and Bristol-Myers-Squibb. J.A.S. has received research support from Bristol Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers-Squibb, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer, and ReCor unrelated to this work. C.M.H. serves in a scientific advisory role for the following companies: Lung Therapeutics, Lassen Therapeutics, Rubedo Life Sciences, and Structure Therapeutics. C.M.H. also consults for the Three Lakes Foundation. C.M.H. receives research funding from Kyowa Kirin Co, Ltd. B.B.M. is a grant review consultant for Boehringer-Ingelheim, the Pulmonary Fibrosis Foundation and the National Scleroderma Foundation. W.M.O. has received consulting fees from Nikang Therapeutics on a topic unrelated to the present manuscript. E.Y.K. receives research funding in fILD from Bayer AG, Roche Pharma Research and Early Development, and 10X Genomics. E.Y.K. has a PCT patent application (US2022/075673) concerning a method to treat fibrosis that is not mentioned in this manuscript. E.Y.K. has a financial interest in Novartis AG unrelated to this work. The funders had no role in the decision to publish or preparation of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health.
Figures

References
-
- Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, Kreuter M, Lynch DA, Maher TM, Martinez FJ, et al. (2022). Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med 205, e18–e47. 10.1164/rccm.202202-0399ST. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

