Dermatology-related quality-of-life outcomes in patients with RAS wild-type metastatic colorectal cancer treated with fluorouracil and folinic acid with or without panitumumab (Pmab) maintenance after FOLFOX + Pmab induction: a prespecified secondary analysis of the phase II randomized PanaMa (AIO KRK 0212) trial
- PMID: 38996519
- PMCID: PMC11452331
- DOI: 10.1016/j.esmoop.2024.103628
Dermatology-related quality-of-life outcomes in patients with RAS wild-type metastatic colorectal cancer treated with fluorouracil and folinic acid with or without panitumumab (Pmab) maintenance after FOLFOX + Pmab induction: a prespecified secondary analysis of the phase II randomized PanaMa (AIO KRK 0212) trial
Abstract
Background: The key endpoints for the assessment of the effect of maintenance therapy for metastatic colorectal cancer (mCRC) are survival and quality-of-life outcomes. We aimed to compare dermatology-related quality of life (DRQOL) in patients with RAS wild-type (wt) mCRC treated with fluorouracil and folinic acid (FU/FA) + panitumumab (Pmab) versus FU/FA alone as maintenance therapy after folinic acid, fluorouracil and oxaliplatin + Pmab induction.
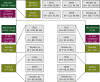
Patients and methods: The phase II randomized PanaMa (AIO KRK 0212; NCT01991873) trial included 387 patients at 70 community/academic sites in Germany. For this prespecified secondary analysis, DRQOL outcomes were assessed using the Functional Assessment of Cancer Therapy-epidermal growth factor receptor inhibitor (FACT-EGFRI), Dermatology Life Quality Index (DLQI), and Skindex-16 questionnaires at every second cycle of therapy until disease progression/death.
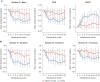
Results: At least one DRQOL questionnaire was completed by a total of 310/377 (82%) patients who received induction therapy, and by 216/248 (87%) patients who were randomized and received maintenance therapy. Patients who experienced skin toxicity according to the National Cancer Institute (NCI)-Common Terminology Criteria for Adverse Events (CTCAE) during induction therapy had significantly worse DRQOL according to all three measures, compared to those who did not [i.e. Skindex-16, mean difference at cycle 2 -12.87; 95% confidence interval (CI) -20.01 to -5.73; P < 0.001]. During maintenance therapy, significantly improved recovery was observed in all DRQOL measures for patients receiving FU/FA, compared to those receiving additional Pmab (i.e. Skindex-16, mean difference at cycle 6 -16.53; 95% CI -22.68 to -10.38; P < 0.001).
Conclusions: In this secondary analysis of a phase II randomized clinical trial, patient-reported DRQOL outcomes correlated with skin toxicity according to NCI-CTCAE during induction therapy. Maintenance therapy with FU/FA + Pmab was associated with deteriorated DRQOL versus FU/FA alone in patients with RAS wt mCRC.
Keywords: anti-EGFR; colorectal cancer; maintenance therapy; patient-reported outcomes; quality of life.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Cervantes A., Adam R., Rosello S., et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10–32. - PubMed
-
- Andre T., Tournigand C., Mineur L., et al. Phase II study of an optimized 5-fluorouracil-oxaliplatin strategy (OPTIMOX2) with celecoxib in metastatic colorectal cancer: a GERCOR study. Ann Oncol. 2007;18(1):77–81. - PubMed
-
- Quidde J., Hegewisch-Becker S., Graeven U., et al. Quality of life assessment in patients with metastatic colorectal cancer receiving maintenance therapy after first-line induction treatment: a preplanned analysis of the phase III AIO KRK 0207 trial. Ann Oncol. 2016;27(12):2203–2210. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

