Improving the hemocompatibility of a porohyperelastic layered vascular graft using luminal reversal microflows
- PMID: 38996626
- PMCID: PMC11513160
- DOI: 10.1016/j.jmbbm.2024.106638
Improving the hemocompatibility of a porohyperelastic layered vascular graft using luminal reversal microflows
Abstract
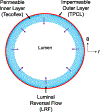
Vascular graft thrombosis is a long-standing clinical problem. A myriad of efforts have been devoted to reducing thrombus formation following bypass surgery. Researchers have primarily taken a chemical approach to engineer and modify surfaces, seeking to make them more suitable for blood contacting applications. Using mechanical forces and surface topology to prevent thrombus formation has recently gained more attention. In this study, we have designed a bilayered porous vascular graft capable of repelling platelets and destabilizing absorbed protein layers from the luminal surface. During systole, fluid penetrates through the graft wall and is subsequently ejected from the wall into the luminal space (Luminal Reversal Flow - LRF), pushing platelets away from the surface during diastole. In-vitro hemocompatibility tests were conducted to compare platelet deposition in high LRF grafts with low LRF grafts. Graft material properties were determined and utilized in a porohyperelastic (PHE) finite element model to computationally predict the LRF generation in each graft type. Hemocompatibility testing showed significantly lower platelet deposition values in high versus low LRF generating grafts (median±IQR = 5,708 ± 987 and 23,039 ± 3,310 platelets per mm2, respectively, p=0.032). SEM imaging of the luminal surface of both graft types confirmed the quantitative blood test results. The computational simulations of high and low LRF generating grafts resulted in LRF values of -10.06 μm/s and -2.87 μm/s, respectively. These analyses show that a 250% increase in LRF is associated with a 75.2% decrease in platelet deposition. PHE vascular grafts with high LRF have the potential to improve anti-thrombogenicity and reduce thrombus-related post-procedure complications. Additional research is required to overcome the limitations of current graft fabrication technologies that further enhance LRF generation.
Keywords: Hemocompatibility; Luminal reversal flow; Polymer biomaterial; Porohyperelastic; Vascular graft.
Copyright © 2024. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jonathan Pieter Vande Geest and Ali Behrangzade has patent #US patent 18008476 pending to University of Pittsburgh. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Optimizing the Porohyperelastic Response of a Layered Compliance Matched Vascular Graft to Promote Luminal Self-Cleaning.J Biomech Eng. 2023 Feb 1;145(2):021002. doi: 10.1115/1.4055563. J Biomech Eng. 2023. PMID: 36082481 Free PMC article.
-
The influence of porosity on the hemocompatibility of polyhedral oligomeric silsesquioxane poly (caprolactone-urea) urethane.Int J Biochem Cell Biol. 2015 Nov;68:176-86. doi: 10.1016/j.biocel.2015.08.007. Epub 2015 Aug 14. Int J Biochem Cell Biol. 2015. PMID: 26279141
-
Luminal surface microgeometry affects platelet adhesion in small-diameter synthetic grafts.Biomaterials. 2004 Aug;25(18):4447-55. doi: 10.1016/j.biomaterials.2003.11.025. Biomaterials. 2004. PMID: 15046935
-
Small caliber vascular grafts. Part II: Polyurethanes revisited.J Biomater Appl. 1996 Jul;11(1):37-61. doi: 10.1177/088532829601100102. J Biomater Appl. 1996. PMID: 8872599 Review.
-
Small caliber vascular grafts. Part I: state of the art.J Biomater Appl. 1996 Apr;10(4):309-29. doi: 10.1177/088532829601000402. J Biomater Appl. 1996. PMID: 8859403 Review.
Cited by
-
A bio-inspired screwed small-diameter vascular graft for endotheliazation and arterial regeneration.Bioact Mater. 2025 Jul 15;53:253-268. doi: 10.1016/j.bioactmat.2025.07.016. eCollection 2025 Nov. Bioact Mater. 2025. PMID: 40697397 Free PMC article.
References
-
- Aavik A, Kibur RT, Lieberg J, Lepner U, Aunapuu M, Arend A, 2019. Cold-stored venous allografts in different preserving solutions: A study on changes in vein wall morphology. Scand. J. Surg. 108 (1), 67–75. - PubMed
-
- Antonova LV, Seifalian AM, Kutikhin AG, Sevostyanova VV, Matveeva VG, Velikanova EA, Mironov AV, Shabaev AR, Glushkova TV, Senokosova EA, Vasyukov GY, Krivkina EO, Burago AY, Kudryavtseva YA, Barbarash OL, Barbarash LS, 2016. Conjugation with RGD peptides and incorporation of vascular endothelial growth factor are equally efficient for biofunctionalization of tissue-engineered vascular grafts. Int. J. Mol. Sci. 17 (11). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

