Effect of ultrasound-mediated blood-spinal cord barrier opening on survival and motor function in females in an amyotrophic lateral sclerosis mouse model
- PMID: 38996764
- PMCID: PMC11284947
- DOI: 10.1016/j.ebiom.2024.105235
Effect of ultrasound-mediated blood-spinal cord barrier opening on survival and motor function in females in an amyotrophic lateral sclerosis mouse model
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by a progressive loss of motor neurons. The limited efficacy of recent therapies in clinical development may be linked to lack of drug penetration to the affected motor neurons due to the blood-brain barrier (BBB) and blood-spinal cord barrier (BSCB).
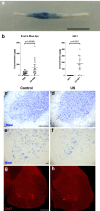
Methods: In this work, the safety and efficacy of repeated short transient opening of the BSCB by low intensity pulsed ultrasound (US, sonication) was studied in females of an ALS mouse model (B6.Cg-Tg(SOD1∗G93A)1Gur/J). The BSCB was disrupted using a 1 MHz ultrasound transducer coupled to the spinal cord, with and without injection of insulin-like growth factor 1 (IGF1), a neurotrophic factor that has previously shown efficacy in ALS models.
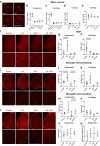
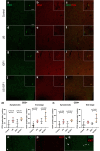
Findings: Results in wild-type (WT) animals demonstrated that the BSCB can be safely disrupted and IGF1 concentrations significantly enhanced after a single session of transient BSCB disruption (176 ± 32 μg/g vs. 0.16 ± 0.008 μg/g, p < 0.0001). Five repeated weekly US sessions performed in female ALS mice demonstrated a survival advantage in mice treated with IGF1 and US (US IGF1) compared to treatment with IGF1 alone (176 vs. 166 days, p = 0.0038). Surprisingly, this survival advantage was also present in mice treated with US alone vs. untreated mice (178.5 vs. 166.5 days, p = 0.0061). Muscle strength did not show difference among the groups. Analysis of glial cell immunoreactivity and microglial transcriptome showing reduced cell proliferation pathways, in addition to lymphocyte infiltration, suggested that the beneficial effect of US or US IGF1 could act through immune cell modulation.
Interpretation: These results show the first step towards a possible beneficial impact of transient BSCB opening for ALS therapy and suggest implication of immune cells.
Funding: Fondation pour la Recherche Médicale (FRM). Investissements d'avenirANR-10-IAIHU-06, Société Française de Neurochirurgie (SFNC), Fond d'étude et de Recherche du Corps Medical (FERCM), Aide à la Recherche des Maladies du Cerveau (ARMC), SLA Fondation Recherche (SLAFR), French Ministry for High Education and Research (MENR), Carthera, Laboratoire de Recherche en Technologies Chirurgicales Avancées (LRTCA).
Keywords: Amyotrophic lateral sclerosis (ALS); Blood-spinal cord barrier (BSCB); Lymphocytes; Motor neuron disease (MND); Neuroinflammation; Ultrasound.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Michael Canney is an employee of Carthera and has ownership interest in the company as well as patents related to this technology. Alexandre Carpentier is a consultant to Carthera, has ownership interest in the company, and has filed patents pertaining to the results presented in the paper. Gaelle Bruneteau: Grants from the French association for ALS (ARSLA) and the French association for Myopathies (AFM), National Hospital Clinical Research Programs (PHRC-N).
Figures



References
-
- Bartanusz V., Jezova D., Alajajian B., et al. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol. 2011;70(2):194–206. - PubMed
-
- Garbuzova-Davis S., Willing A.E., Zigova T., et al. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J Hematother Stem Cell Res. 2003;12(3):255–270. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

