Combination cell therapy leads to clonal deletion of donor-specific T cells in kidney transplant recipients
- PMID: 38996766
- PMCID: PMC11284950
- DOI: 10.1016/j.ebiom.2024.105239
Combination cell therapy leads to clonal deletion of donor-specific T cells in kidney transplant recipients
Abstract
Background: Induction of donor-specific tolerance is a promising approach to achieve long-term graft patency in transplantation with little to no maintenance immunosuppression. Changes to the recipient's T cell receptor (TCR) repertoire are understood to play a pivotal role in the establishment of a robust state of tolerance in chimerism-based transplantation protocols.
Methods: We investigated changes to the TCR repertoires of patients participating in an ongoing prospective, controlled, phase I/IIa trial designed to evaluate the safety and efficacy of combination cell therapy in living donor kidney transplantation. Using high-throughput sequencing, we characterized the repertoires of six kidney recipients who also received bone marrow from the same donor (CKBMT), together with an infusion of polyclonal autologous Treg cells instead of myelosuppression.
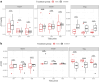
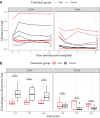
Findings: Patients undergoing combination cell therapy exhibited partial clonal deletion of donor-reactive CD4+ T cells at one, three, and six months post-transplant, compared to control patients receiving the same immunosuppression regimen but no cell therapy (p = 0.024). The clonality, R20 and turnover rates of the CD4+ and CD8+ TCR repertoires were comparable in both groups, showing our protocol caused no excessive repertoire shift or loss of diversity. Treg clonality was lower in the case group than in control (p = 0.033), suggesting combination cell therapy helps to preserve Treg diversity.
Interpretation: Overall, our data indicate that combining Treg cell therapy with CKBMT dampens the alloimmune response to transplanted kidneys in humans in the absence of myelosuppression.
Funding: This study was funded by the Vienna Science and Technology Fund (WWTF).
Keywords: Alloreactivity; Cell therapy; Immunological tolerance; Kidney transplantation; T cell receptor.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have declared that no conflict of interest exists.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

