Impact of pre-transplant immune checkpoint inhibitor use on post-transplant outcomes in HCC: A systematic review and individual patient data meta-analysis
- PMID: 38996924
- PMCID: PMC11655254
- DOI: 10.1016/j.jhep.2024.06.042
Impact of pre-transplant immune checkpoint inhibitor use on post-transplant outcomes in HCC: A systematic review and individual patient data meta-analysis
Abstract
Background & aims: Treatment with immune checkpoint inhibitors (ICIs) for hepatocellular carcinoma (HCC) prior to liver transplantation (LT) has been reported; however, ICIs may elevate the risk of allograft rejection and impact other clinical outcomes. This study aims to summarize the impact of ICI use on post-LT outcomes.
Methods: In this individual patient data meta-analysis, we searched databases to identify HCC cases treated with ICIs before LT, detailing allograft rejection, HCC recurrence, and overall survival. We performed Cox regression analysis to identify risk factors for allograft rejection.
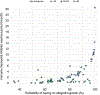
Results: Among 91 eligible patients, with a median (IQR) follow-up of 690.0 (654.5) days, there were 24 (26.4%) allograft rejections, 9 (9.9%) HCC recurrences, and 9 (9.9%) deaths. Age (adjusted hazard ratio [aHR] per 10 years 0.72, 95% CI 0.53-0.99, p = 0.044) and ICI washout time (aHR per 1 week 0.92, 95% CI 0.86-0.99, p = 0.022) were associated with allograft rejection. The median (IQR) washout period for patients with ≤20% probability of allograft rejection was 94 (196) days. Overall survival did not differ between cases with and without allograft rejection (log-rank test, p = 0.2). Individuals with HCC recurrence had fewer median (IQR) ICI cycles than those without recurrence (4.0 [1.8] vs. 8.0 [9.0]; p = 0.025). The proportion of patients within Milan post-ICI was lower for those with recurrence vs. without (16.7% vs. 65.3%, p = 0.032).
Conclusion: Patients have acceptable post-LT outcomes after ICI therapy. Age and ICI washout length relate to the allograft rejection risk, and a 3-month washout may reduce it to that of patients without ICI exposure. Number of ICI cycles and tumor burden may affect recurrence risk. Large prospective studies are necessary to confirm these associations.
Impact and implications: This systematic review and individual patient data meta-analysis of 91 patients with hepatocellular carcinoma and immune checkpoint inhibitor use prior to liver transplantation suggest acceptable overall post-transplant outcomes. Older age and longer immune checkpoint inhibitor washout period have a significant inverse association with the risk of allograft rejection. A 3-month washout may reduce it to that of patients without immune checkpoint inhibitor exposure. Additionally, a higher number of immune checkpoint inhibitor cycles and tumor burden within Milan criteria at the completion of immunotherapy may predict a decreased risk of hepatocellular carcinoma recurrence, but this observation requires further validation in larger prospective studies.
Keywords: Graft Rejection; Hepatocellular Carcinoma; Immune Checkpoint Inhibitors; Liver Neoplasms; Liver Transplantation; Recurrence.
Copyright © 2024 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
Ju Dong Yang provides a consulting service for AstraZeneca, Eisai, Exact Sciences, Exelixis, Fujifilm Medical Sciences, and Gilead Sciences. Neehar Parikh has served as a consultant or advisor for Genentech, Fujifilm Medical, Eisai, Exelixis, Merck, Exact Sciences, Freenome, and Gilead. Amit Singal has served as a consultant or on advisory boards for Genentech, AstraZeneca, Bayer, Eisai, Exelixis, Merck, Elevar, Boston Scientific, Sirtex, HistoSonics, Fujifilm Medical Sciences, Exact Sciences, Glycotest, Abbott, Roche, Freenome, and GRAIL. Neil Mehta has served as a consultant or advisor for Exelixis, Fujifilm Medical, Genentech, Eisai, Exelixis, Exact Sciences, and Merck. Sherrie Bhoori serves as an advisor or lecturer for Roche, AstraZeneca, Boston Scientific, Terumo. Beau B. Toskich serves as an advisor for Genentech, Eisai, and Astra Zeneca. Robyn D. Gartrell’s laboratory receives funding from Hyundai Hope on Wheels Hope Scholar Award, Swim Across America, Rally Foundation, StacheStrong and Musella Foundation. Bruno Sangro reports consulting or advisory fees from AstraZeneca, Bayer, Boston Scientific, Bristol-Myers Squibb, Eisai, lncyte, IPSEN, Roche, Sirtex Medical, and Terumo; reports being an invited speaker for AstraZeneca, Bristol-Myers Squibb, Eisai, lncyte, IPSEN, Roche, and Sirtex Medical; research funding (to institution) from Bristol-Myers Squibb and Sirtex Medical. Tarek Hassanein serves as an advisory committee member or review panelist for AbbVie, Cymabay, Gilead, HepQuant, Madrigal, Mallinckrodt; has received grant and research support from AbbVie, Amgen, Biolinq, Bristol-Myers Squibb, Astra Zeneca, Boehringerlngelheim, Bristol-Myers Squibb, COUR, DURECT Corporation, Escient, Galectin, Gilead, Grifols, HepQuant, Intercept, Janssen, Merck, Mirum, NeuroBo, Novartis, Novo Nordisk, Pfizer, Regeneron, Salix Pharmaceuticals, Sonic lncytes. Takeda, Terns Pharmaceuticals, Valeant; and also involved in speaking engagements and teaching for for AbbVie, Gilead, Intercept, Mallinckrodt, Salix Pharmaceuticals. Davendra Sohal has served on the speakers Bureau for Astra Zeneca since January 2024, lncyte since January 2021, and Seagen since January 2023; and has received consulting fees or honoraria from Astra Zeneca (ended Jan 2024), Replimune (ended Jan 2024), Cancer Commons (ended Jun 2023), TransThera (ended Jun 2022), Totus Medicines (ended Jul 2023), Valar Labs (ended Dec 2022), Aadi (ended Jun 2023), Elevar, Regeneron; and has received research funding from Aadi, Ability Pharma, Amgen, Apexigen, Astellas, Astra Zeneca, Bexion, Bristol-Myers Squibb, FibroGen, Genentech, Hengrui, Merck, Mirati, NextCure, PanCAN, Regeneron, Roche, Triumvira. Nguyen H Tran has served as an advisor for Astrazeneca, Genentech, Helsinn and TEMPUS. She is a recipient of the K23MD017217–01A1. Parissa Tabrizian serves as an advisor for Bayer. Astrazeneca, boston scientific. -honorarium. Mehmet Akce has been involved in research projects with Bristol-Myers Squibb-Ono Pharmaceutical (Inst), Xencor (Inst), Merck Sharp & Dohme (Inst), Eisai (Inst), GSK (Inst), Bayer (Inst), Relay (Inst), ProDa BioTech (Inst), Exelixis (lnst),and AstraZeneca (Inst) and also has consulting or advisory roles for Eisai, Ipsen, Exelixis, GSK, QED, lsofol, Curio Science, AstraZeneca, Genentech, lncyte, and Taiho. Other authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- Lucey MR, Furuya KN. Foley DP. Liver transplantation. New Engl J Med 2023;389:1888–1900. - PubMed
-
- Matevish L, Patel MS, Vagefi PA. Downstaging techniques for hepatocellular carcinoma in candidates awaiting liver transplantation. Surg Clin North America 2023;104(1):145–162. - PubMed
-
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl 2002;8:765–774. - PubMed

