Artificial intelligence system for outcome evaluations of human in vitro fertilization-derived embryos
- PMID: 38997251
- PMCID: PMC11332789
- DOI: 10.1097/CM9.0000000000003162
Artificial intelligence system for outcome evaluations of human in vitro fertilization-derived embryos
Abstract
Background: In vitro fertilization (IVF) has emerged as a transformative solution for infertility. However, achieving favorable live-birth outcomes remains challenging. Current clinical IVF practices in IVF involve the collection of heterogeneous embryo data through diverse methods, including static images and temporal videos. However, traditional embryo selection methods, primarily reliant on visual inspection of morphology, exhibit variability and are contingent on the experience of practitioners. Therefore, an automated system that can evaluate heterogeneous embryo data to predict the final outcomes of live births is highly desirable.
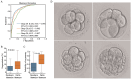
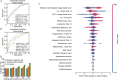
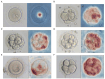
Methods: We employed artificial intelligence (AI) for embryo morphological grading, blastocyst embryo selection, aneuploidy prediction, and final live-birth outcome prediction. We developed and validated the AI models using multitask learning for embryo morphological assessment, including pronucleus type on day 1 and the number of blastomeres, asymmetry, and fragmentation of blastomeres on day 3, using 19,201 embryo photographs from 8271 patients. A neural network was trained on embryo and clinical metadata to identify good-quality embryos for implantation on day 3 or day 5, and predict live-birth outcomes. Additionally, a 3D convolutional neural network was trained on 418 time-lapse videos of preimplantation genetic testing (PGT)-based ploidy outcomes for the prediction of aneuploidy and consequent live-birth outcomes.
Results: These two approaches enabled us to automatically assess the implantation potential. By combining embryo and maternal metrics in an ensemble AI model, we evaluated live-birth outcomes in a prospective cohort that achieved higher accuracy than experienced embryologists (46.1% vs. 30.7% on day 3, 55.0% vs. 40.7% on day 5). Our results demonstrate the potential for AI-based selection of embryos based on characteristics beyond the observational abilities of human clinicians (area under the curve: 0.769, 95% confidence interval: 0.709-0.820). These findings could potentially provide a noninvasive, high-throughput, and low-cost screening tool to facilitate embryo selection and achieve better outcomes.
Conclusions: Our study underscores the AI model's ability to provide interpretable evidence for clinicians in assisted reproduction, highlighting its potential as a noninvasive, efficient, and cost-effective tool for improved embryo selection and enhanced IVF outcomes. The convergence of cutting-edge technology and reproductive medicine has opened new avenues for addressing infertility challenges and optimizing IVF success rates.
Copyright © 2024 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures



Similar articles
-
Development of an artificial intelligence model for predicting the likelihood of human embryo euploidy based on blastocyst images from multiple imaging systems during IVF.Hum Reprod. 2022 Jul 30;37(8):1746-1759. doi: 10.1093/humrep/deac131. Hum Reprod. 2022. PMID: 35674312 Free PMC article.
-
Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation.Cochrane Database Syst Rev. 2020 Sep 8;9(9):CD005291. doi: 10.1002/14651858.CD005291.pub3. Cochrane Database Syst Rev. 2020. PMID: 32898291 Free PMC article.
-
Development and evaluation of a live birth prediction model for evaluating human blastocysts from a retrospective study.Elife. 2023 Feb 22;12:e83662. doi: 10.7554/eLife.83662. Elife. 2023. PMID: 36810139 Free PMC article.
-
Cumulative live birth rates of 31 478 untested embryos from 11 463 women challenge traditional recurrent implantation failure definitions.Hum Reprod. 2025 May 1;40(5):818-833. doi: 10.1093/humrep/deaf036. Hum Reprod. 2025. PMID: 40064027
-
Noninvasive genetic screening: current advances in artificial intelligence for embryo ploidy prediction.Fertil Steril. 2023 Aug;120(2):228-234. doi: 10.1016/j.fertnstert.2023.06.025. Epub 2023 Jun 30. Fertil Steril. 2023. PMID: 37394089 Review.
Cited by
-
Non-invasive prediction of human embryonic ploidy using artificial intelligence: a systematic review and meta-analysis.EClinicalMedicine. 2024 Oct 24;77:102897. doi: 10.1016/j.eclinm.2024.102897. eCollection 2024 Nov. EClinicalMedicine. 2024. PMID: 39513188 Free PMC article.
References
-
- Baxter Bendus AE, Mayer JF, Shipley SK, Catherino WH. Interobserver and intraobserver variation in day 3 embryo grading. Fertil Steril 2006;86:1608–1615. doi: 10.1016/j.fertnstert.2006.05.037. - PubMed
-
- Storr A, Venetis CA, Cooke S, Kilani S, Ledger W. Inter-observer and intra-observer agreement between embryologists during selection of a single Day 5 embryo for transfer: a multicenter study. Hum Reprod 2017;32:307–314. doi: 10.1093/humrep/dew330. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

