Priming antibody responses to the fusion peptide in rhesus macaques
- PMID: 38997302
- PMCID: PMC11245479
- DOI: 10.1038/s41541-024-00918-9
Priming antibody responses to the fusion peptide in rhesus macaques
Abstract
Immunodominance of antibodies targeting non-neutralizing epitopes and the high level of somatic hypermutation within germinal centers (GCs) required for most HIV broadly neutralizing antibodies (bnAbs) are major impediments to the development of an effective HIV vaccine. Rational protein vaccine design and non-conventional immunization strategies are potential avenues to overcome these hurdles. Here, we report using implantable osmotic pumps to continuously deliver a series of epitope-targeted immunogens to rhesus macaques over the course of six months to prime and elicit antibody responses against the conserved fusion peptide (FP). GC responses and antibody specificities were tracked longitudinally using lymph node fine-needle aspirates and electron microscopy polyclonal epitope mapping (EMPEM), respectively, to show antibody responses to the FP/N611 glycan hole region were primed, although exhibited limited neutralization breadth. Application of cryoEMPEM delineated key residues for on-target and off-target responses that can drive the next round of structure-based vaccine design.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
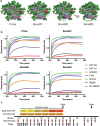
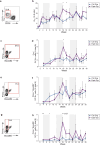

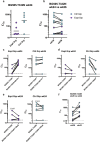
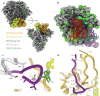
Update of
-
Focusing antibody responses to the fusion peptide in rhesus macaques.bioRxiv [Preprint]. 2023 Jun 27:2023.06.26.545779. doi: 10.1101/2023.06.26.545779. bioRxiv. 2023. Update in: NPJ Vaccines. 2024 Jul 12;9(1):126. doi: 10.1038/s41541-024-00918-9. PMID: 37425865 Free PMC article. Updated. Preprint.
Similar articles
-
Focusing antibody responses to the fusion peptide in rhesus macaques.bioRxiv [Preprint]. 2023 Jun 27:2023.06.26.545779. doi: 10.1101/2023.06.26.545779. bioRxiv. 2023. Update in: NPJ Vaccines. 2024 Jul 12;9(1):126. doi: 10.1038/s41541-024-00918-9. PMID: 37425865 Free PMC article. Updated. Preprint.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
HIV-1 Subtype C-Infected Children with Exceptional Neutralization Breadth Exhibit Polyclonal Responses Targeting Known Epitopes.J Virol. 2018 Aug 16;92(17):e00878-18. doi: 10.1128/JVI.00878-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29950423 Free PMC article.
-
Structure-guided envelope trimer design in HIV-1 vaccine development: a narrative review.J Int AIDS Soc. 2021 Nov;24 Suppl 7(Suppl 7):e25797. doi: 10.1002/jia2.25797. J Int AIDS Soc. 2021. PMID: 34806305 Free PMC article. Review.
-
Strategies for induction of HIV-1 envelope-reactive broadly neutralizing antibodies.J Int AIDS Soc. 2021 Nov;24 Suppl 7(Suppl 7):e25831. doi: 10.1002/jia2.25831. J Int AIDS Soc. 2021. PMID: 34806332 Free PMC article. Review.
Cited by
-
Immunofocusing on the conserved fusion peptide of HIV envelope glycoprotein in rhesus macaques.bioRxiv [Preprint]. 2025 Jun 5:2024.11.27.625755. doi: 10.1101/2024.11.27.625755. bioRxiv. 2025. PMID: 39651156 Free PMC article. Preprint.
-
Vaccination with mRNA-encoded membrane-bound HIV Envelope trimer induces neutralizing antibodies in animal models.bioRxiv [Preprint]. 2025 Jan 25:2025.01.24.634423. doi: 10.1101/2025.01.24.634423. bioRxiv. 2025. Update in: Sci Transl Med. 2025 Jul 30;17(809):eadw0721. doi: 10.1126/scitranslmed.adw0721. PMID: 39896562 Free PMC article. Updated. Preprint.
References
-
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization – recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2011;60:1–64. - PubMed
Grants and funding
- UM1 AI144462/AI/NIAID NIH HHS/United States
- UM1 AI100663/AI/NIAID NIH HHS/United States
- P01 AI048240/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- AI048240/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- U24 GM129547/GM/NIGMS NIH HHS/United States
- U42 OD011023/OD/NIH HHS/United States
- AI100663/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- R01 AI145629/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- AI144462/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- Al131873/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
LinkOut - more resources
Full Text Sources
Miscellaneous

