Retinal hyperspectral imaging in mouse models of Parkinson's disease and healthy aging
- PMID: 38997314
- PMCID: PMC11245556
- DOI: 10.1038/s41598-024-66284-7
Retinal hyperspectral imaging in mouse models of Parkinson's disease and healthy aging
Abstract
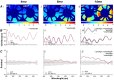
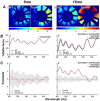
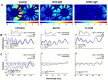
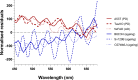
Retinal hyperspectral imaging (HSI) is a non-invasive in vivo approach that has shown promise in Alzheimer's disease. Parkinson's disease is another neurodegenerative disease where brain pathobiology such as alpha-synuclein and iron overaccumulation have been implicated in the retina. However, it remains unknown whether HSI is altered in in vivo models of Parkinson's disease, whether it differs from healthy aging, and the mechanisms which drive these changes. To address this, we conducted HSI in two mouse models of Parkinson's disease across different ages; an alpha-synuclein overaccumulation model (hA53T transgenic line M83, A53T) and an iron deposition model (Tau knock out, TauKO). In comparison to wild-type littermates the A53T and TauKO mice both demonstrated increased reflectivity at short wavelengths ~ 450 to 600 nm. In contrast, healthy aging in three background strains exhibited the opposite effect, a decreased reflectance in the short wavelength spectrum. We also demonstrate that the Parkinson's hyperspectral signature is similar to that from an Alzheimer's disease model, 5xFAD mice. Multivariate analyses of HSI were significant when plotted against age. Moreover, when alpha-synuclein, iron or retinal nerve fibre layer thickness were added as a cofactor this improved the R2 values of the correlations in certain groups. This study demonstrates an in vivo hyperspectral signature in Parkinson's disease that is consistent in two mouse models and is distinct from healthy aging. There is also a suggestion that factors including retinal deposition of alpha-synuclein and iron may play a role in driving the Parkinson's disease hyperspectral profile and retinal nerve fibre layer thickness in advanced aging. These findings suggest that HSI may be a promising translation tool in Parkinson's disease.
Keywords: A53T; Ageing; Alzheimer’s disease; Hyperspectral; Parkinson’s disease; TauKO.
© 2024. The Author(s).
Conflict of interest statement
CN and BB are joint investigators on an Australian Research Council Linkage grant LP160100126 with AstraZeneca Neuroscience and Biogen Inc. No conflicts of interest, financial or otherwise, are declared by the other authors.
Figures


Similar articles
-
Accelerated accumulation of retinal α-synuclein (pSer129) and tau, neuroinflammation, and autophagic dysregulation in a seeded mouse model of Parkinson's disease.Neurobiol Dis. 2019 Jan;121:1-16. doi: 10.1016/j.nbd.2018.09.013. Epub 2018 Sep 12. Neurobiol Dis. 2019. PMID: 30218757
-
Retina as a Model to Study In Vivo Transmission of α-Synuclein in the A53T Mouse Model of Parkinson's Disease.Methods Mol Biol. 2021;2224:75-85. doi: 10.1007/978-1-0716-1008-4_5. Methods Mol Biol. 2021. PMID: 33606207
-
Retinal hyperspectral imaging in the 5xFAD mouse model of Alzheimer's disease.Sci Rep. 2021 Mar 18;11(1):6387. doi: 10.1038/s41598-021-85554-2. Sci Rep. 2021. PMID: 33737550 Free PMC article.
-
Retinal α-synuclein deposits in Parkinson's disease patients and animal models.Acta Neuropathol. 2019 Mar;137(3):379-395. doi: 10.1007/s00401-018-01956-z. Epub 2019 Feb 5. Acta Neuropathol. 2019. PMID: 30721408 Review.
-
Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder?Mov Disord. 2013 Jan;28(1):31-40. doi: 10.1002/mds.25373. Mov Disord. 2013. PMID: 23390095 Review.
References
-
- Chang CI. Hyperspectral Imaging: Techniques for Spectral Detection and Classification. Kluwer Academic/Plenum Publishers; 2003.
-
- Siderowf A, Concha-Marambio L, Lafontant DE, Farris CM, Ma Y, Urenia PA, Nguyen H, Alcalay RN, Chahine LM, Foroud T, et al. Assessment of heterogeneity among participants in the Parkinson’s progression markers initiative cohort using alpha-synuclein seed amplification: A cross-sectional study. Lancet Neurol. 2023;22:407–417. doi: 10.1016/S1474-4422(23)00109-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

