Neutrophil-derived migrasomes are an essential part of the coagulation system
- PMID: 38997457
- PMCID: PMC11251984
- DOI: 10.1038/s41556-024-01440-9
Neutrophil-derived migrasomes are an essential part of the coagulation system
Abstract
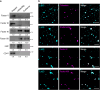
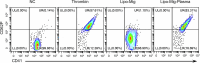
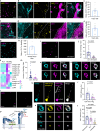
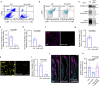
Migrasomes are organelles that are generated by migrating cells. Here we report the key role of neutrophil-derived migrasomes in haemostasis. We found that a large number of neutrophil-derived migrasomes exist in the blood of mice and humans. Compared with neutrophil cell bodies and platelets, these migrasomes adsorb and enrich coagulation factors on the surface. Moreover, they are highly enriched with adhesion molecules, which enable them to preferentially accumulate at sites of injury, where they trigger platelet activation and clot formation. Depletion of neutrophils, or genetic reduction of the number of these migrasomes, significantly decreases platelet plug formation and impairs coagulation. These defects can be rescued by intravenous injection of purified neutrophil-derived migrasomes. Our study reveals neutrophil-derived migrasomes as a previously unrecognized essential component of the haemostasis system, which may shed light on the cause of various coagulation disorders and open therapeutic possibilities.
© 2024. The Author(s).
Conflict of interest statement
L.Y. is the scientific founder of Migrasome Therapeutics. The remaining authors declare no competing interests.
Figures









References
-
- Mussbacher, M., Kral-Pointner, J. B., Salzmann, M., Schrottmaier, W. C. & Assinger, A. in Fundamentals of Vascular Biology (ed. Geiger, M.) 145–169 (Springer International Publishing, 2019).
Publication types
MeSH terms
Substances
Grants and funding
- 32030023/National Natural Science Foundation of China (National Science Foundation of China)
- 92354306/National Natural Science Foundation of China (National Science Foundation of China)
- 32330025/National Natural Science Foundation of China (National Science Foundation of China)
- 82173246/National Natural Science Foundation of China (National Science Foundation of China)
- 2019M660611/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources

