Bacterial reprogramming of tick metabolism impacts vector fitness and susceptibility to infection
- PMID: 38997520
- PMCID: PMC11926704
- DOI: 10.1038/s41564-024-01756-0
Bacterial reprogramming of tick metabolism impacts vector fitness and susceptibility to infection
Abstract
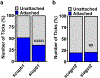
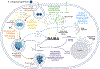
Arthropod-borne pathogens are responsible for hundreds of millions of infections in humans each year. The blacklegged tick, Ixodes scapularis, is the predominant arthropod vector in the United States and is responsible for transmitting several human pathogens, including the Lyme disease spirochete Borrelia burgdorferi and the obligate intracellular rickettsial bacterium Anaplasma phagocytophilum, which causes human granulocytic anaplasmosis. However, tick metabolic response to microbes and whether metabolite allocation occurs upon infection remain unknown. Here we investigated metabolic reprogramming in the tick ectoparasite I. scapularis and determined that the rickettsial bacterium A. phagocytophilum and the spirochete B. burgdorferi induced glycolysis in tick cells. Surprisingly, the endosymbiont Rickettsia buchneri had a minimal effect on bioenergetics. An unbiased metabolomics approach following A. phagocytophilum infection of tick cells showed alterations in carbohydrate, lipid, nucleotide and protein metabolism, including elevated levels of the pleiotropic metabolite β-aminoisobutyric acid. We manipulated the expression of genes associated with β-aminoisobutyric acid metabolism in I. scapularis, resulting in feeding impairment, diminished survival and reduced bacterial acquisition post haematophagy. Collectively, we discovered that metabolic reprogramming affects interspecies relationships and fitness in the clinically relevant tick I. scapularis.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures









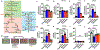


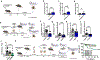
Update of
-
Metabolic disruption impacts tick fitness and microbial relationships.bioRxiv [Preprint]. 2023 May 26:2023.05.26.542501. doi: 10.1101/2023.05.26.542501. bioRxiv. 2023. Update in: Nat Microbiol. 2024 Sep;9(9):2278-2291. doi: 10.1038/s41564-024-01756-0. PMID: 37292783 Free PMC article. Updated. Preprint.
References
-
- WHO. Vector-borne diseases, https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (2020).
-
- Smith RP Tick-borne diseases of humans. Emerg Infect Dis 11, 1808–1809 (2005).
MeSH terms
Grants and funding
- F31 AI152215/AI/NIAID NIH HHS/United States
- F31AI152215/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P01AI138949/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- T32 AI162579/AI/NIAID NIH HHS/United States
- T32AI162579/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI093653/AI/NIAID NIH HHS/United States
- R01 AI080615/AI/NIAID NIH HHS/United States
- P01 AI138949/AI/NIAID NIH HHS/United States
- S10 OD025101/OD/NIH HHS/United States
- R01AI162819/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P01AI138949, R01AI080615/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R21 AI165520/AI/NIAID NIH HHS/United States
- R01 AI116523/AI/NIAID NIH HHS/United States
- F31AI167471/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI049424/AI/NIAID NIH HHS/United States
- F31 AI167471/AI/NIAID NIH HHS/United States
- R01AI134696, R01AI116523, R01AI049424, P01AI138949/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI134696/AI/NIAID NIH HHS/United States
- R01 AI162819/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

