Precise in vivo RNA base editing with a wobble-enhanced circular CLUSTER guide RNA
- PMID: 38997581
- PMCID: PMC11994451
- DOI: 10.1038/s41587-024-02313-0
Precise in vivo RNA base editing with a wobble-enhanced circular CLUSTER guide RNA
Abstract
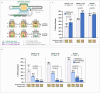
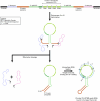
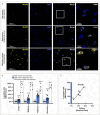
Recruiting the endogenous editing enzyme adenosine deaminase acting on RNA (ADAR) with tailored guide RNAs for adenosine-to-inosine (A-to-I) RNA base editing is promising for safely manipulating genetic information at the RNA level. However, the precision and efficiency of editing are often compromised by bystander off-target editing. Here, we find that in 5'-UAN triplets, which dominate bystander editing, G•U wobble base pairs effectively mitigate off-target events while maintaining high on-target efficiency. This strategy is universally applicable to existing A-to-I RNA base-editing systems and complements other suppression methods such as G•A mismatches and uridine (U) depletion. Combining wobble base pairing with a circularized format of the CLUSTER approach achieves highly precise and efficient editing (up to 87%) of a disease-relevant mutation in the Mecp2 transcript in cell culture. Virus-mediated delivery of the guide RNA alone realizes functional MeCP2 protein restoration in the central nervous system of a murine Rett syndrome model with editing yields of up to 19% and excellent bystander control in vivo.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: T.S., G.M. N.W., C.P.W., L.S.P., A.E.S. and P.R. hold patents on site-directed RNA editing. T.S., C.P.W., A.E.S. and P.R. are inventors of a filed patent based on the work published here. T.S. is a cofounder and shareholder of AIRNA Bio. G.M. is a cofounder and shareholder of Vico Therapeutics. The other authors declare no conflicts of interest.
Figures













References
-
- Stafforst, T. & Schneider, M. F. An RNA–deaminase conjugate selectively repairs point mutations. Angew. Chem. Int. Ed. Engl.51, 11166–11169 (2012). - PubMed
MeSH terms
Substances
Grants and funding
- 2039/20/Israel Science Foundation (ISF)
- IG-Reauts-2019-05/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 96876/Volkswagen Foundation (VolkswagenStiftung)
- 3806/International Rett Syndrome Foundation (IRSF)
- NS110868/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

