Biophysical characterization of the phase separation of TDP-43 devoid of the C-terminal domain
- PMID: 38997630
- PMCID: PMC11245819
- DOI: 10.1186/s11658-024-00615-4
Biophysical characterization of the phase separation of TDP-43 devoid of the C-terminal domain
Abstract
Background: Frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-TDP), amyotrophic lateral sclerosis (ALS) and limbic-predominant age-related TDP-43 encephalopathy (LATE) are associated with deposition of cytoplasmic inclusions of TAR DNA-binding protein 43 (TDP-43) in neurons. One complexity of this process lies in the ability of TDP-43 to form liquid-phase membraneless organelles in cells. Previous work has shown that the recombinant, purified, prion-like domain (PrLD) forms liquid droplets in vitro, but the behaviour of the complementary fragment is uncertain.
Methods: We have purified such a construct without the PrLD (PrLD-less TDP-43) and have induced its phase separation using a solution-jump method and an array of biophysical techniques to study the morphology, state of matter and structure of the TDP-43 assemblies.
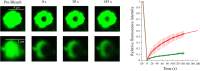
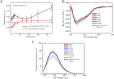
Results: The fluorescent TMR-labelled protein construct, imaged using confocal fluorescence, formed rapidly (< 1 min) round, homogeneous and 0.5-1.0 µm wide assemblies which then coalesced into larger, yet round, species. When labelled with AlexaFluor488, they initially exhibited fluorescence recovery after photobleaching (FRAP), showing a liquid behaviour distinct from full-length TDP-43 and similar to PrLD. The protein molecules did not undergo major structural changes, as determined with circular dichroism and intrinsic fluorescence spectroscopies. This process had a pH and salt dependence distinct from those of full-length TDP-43 and its PrLD, which can be rationalized on the grounds of electrostatic forces.
Conclusions: Similarly to PrLD, PrLD-less TDP-43 forms liquid droplets in vitro through liquid-liquid phase separation (LLPS), unlike the full-length protein that rather undergoes liquid-solid phase separation (LSPS). These results offer a rationale of the complex electrostatic forces governing phase separation of full-length TDP-43 and its fragments. On the one hand, PrLD-less TDP-43 has a low pI and oppositively charged domains, and LLPS is inhibited by salts, which attenuate inter-domain electrostatic attractions. On the other hand, PrLD is positively charged due to a high isoionic point (pI) and LLPS is therefore promoted by salts and pH increases as they both reduce electrostatic repulsions. By contrast, full-length TDP-43 undergoes LSPS most favourably at its pI, with positive and negative salt dependences at lower and higher pH, respectively, depending on whether repulsive or attractive forces dominate, respectively.
Keywords: Electrostatics; LLPS; Liquid–liquid phase separation; Liquid–solid phase separation; Motor neuron diseases; RNA-binding proteins; Self-assembly.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative in-clusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006 doi: 10.1016/j.bbrc.2006.10.093. - DOI - PubMed
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006 doi: 10.1126/science.1134108. - DOI - PubMed
-
- Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C, Coyle-Gilchrist ITS, Chui HC, Fardo DW, Flanagan ME, Halliday G, Hokkanen SRK, Hunter S, Jicha GA, Katsumata Y, Kawas CH, Keene CD, Kovacs GG, Kukull WA, Levey AI, Makkinejad N, Montine TJ, Murayama S, Murray ME, Nag S, Rissman RA, Seeley WW, Sperling RA, White CL, 3rd, Yu L, Schneider JA. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019 doi: 10.1093/brain/awz099. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

