Longitudinal lung function trajectories in response to azithromycin therapy for chronic lung disease in children with HIV infection: a secondary analysis of the BREATHE trial
- PMID: 38997676
- PMCID: PMC11245797
- DOI: 10.1186/s12890-024-03155-x
Longitudinal lung function trajectories in response to azithromycin therapy for chronic lung disease in children with HIV infection: a secondary analysis of the BREATHE trial
Abstract
Background: Chronic lung disease (CLD) is common among children with HIV (CWH) including in those taking antiretroviral therapy (ART). Azithromycin has both antimicrobial and anti-inflammatory effects and has been effective in improving lung function in a variety of lung diseases. We investigated lung function trajectories among CWH with CLD on ART enrolled in a randomized controlled trial of adjuvant azithromycin. We also investigated factors that modified the effect of azithromycin on lung function.
Methods: The study used data from a double-blinded placebo-controlled trial conducted in Malawi and Zimbabwe of 48 weeks on azithromycin (BREATHE: ClinicalTrials.gov NCT02426112) among CWH aged 6 to 19 years taking ART for at least six months who had a forced expiratory volume in one second (FEV1) z-score <-1.0. Participants had a further follow-up period of 24 weeks after intervention cessation. FEV1, forced vital capacity (FVC) and FEV1/FVC were measured at baseline, 24, 48 and 72-weeks and z-scores values calculated. Generalized estimating equations (GEE) models were used to determine the mean effect of azithromycin on lung-function z-scores at each follow-up time point.
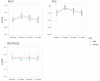
Results: Overall, 347 adolescents (51% male, median age 15 years) were randomized to azithromycin or placebo. The median duration on ART was 6.2 (interquartile range: 3.8-8.6) years and 56.2% had an HIV viral load < 1000copies/ml at baseline. At baseline, the mean FEV1 z-score was - 2.0 (0.7) with 44.7% (n = 155) having an FEV1 z-score <-2, and 10.1% had microbiological evidence of azithromycin resistance. In both trial arms, FEV1 and FVC z-scores improved by 24 weeks but appeared to decline thereafter. The adjusted overall mean difference in FEV1 z-score between the azithromycin and placebo arms was 0.004 [-0.08, 0.09] suggesting no azithromycin effect and this was similar for other lung function parameters. There was no evidence of interaction between azithromycin effect and baseline age, lung function, azithromycin resistance or HIV viral load.
Conclusion: There was no observed azithromycin effect on lung function z-scores at any time point suggesting no therapeutic effect on lung function.
Trial registration: ClinicalTrials.gov NCT02426112. First registered on 24/04/2015.
Keywords: Adolescents; Africa; Azithromycin; Children; Chronic lung disease; FEV1; HIV.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- World AIDS, UNICEF DATA. Day Report 2020 [Internet]. 2020 [cited 2021 Jul 16]. https://data.unicef.org/resources/world-aids-day-report-2020/.
-
- Mutanga JN, Mutembo S, Ezeamama AE, Song X, Fubisha RC, Mutesu-Kapembwa K, et al. Long-term survival outcomes of HIV infected children receiving antiretroviral therapy: an observational study from Zambia (2003–2015) BMC Public Health. 2019;19(1):115. doi: 10.1186/s12889-019-6444-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

