Application of CuNPs and AMF alleviates arsenic stress by encompassing reduced arsenic uptake through metabolomics and ionomics alterations in Elymus sibiricus
- PMID: 38997682
- PMCID: PMC11245830
- DOI: 10.1186/s12870-024-05359-z
Application of CuNPs and AMF alleviates arsenic stress by encompassing reduced arsenic uptake through metabolomics and ionomics alterations in Elymus sibiricus
Abstract
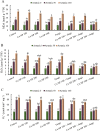
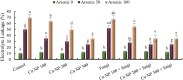
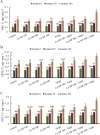
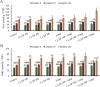
Recent studies have exhibited a very promising role of copper nanoparticles (CuNPs) in mitigation of abiotic stresses in plants. Arbuscular mycorrhizae fungi (AMF) assisted plants to trigger their defense mechanism against abiotic stresses. Arsenic (As) is a non-essential and injurious heavy-metal contaminant. Current research work was designed to elucidate role of CuNPs (100, 200 and 300 mM) and a commercial inoculum of Glomus species (Clonex® Root Maximizer) either alone or in combination (CuNPs + Clonex) on physiology, growth, and stress alleviation mechanisms of E. sibiricus growing in As spiked soils (0, 50, and 100 mg Kg- 1 soil). Arsenic induced oxidative stress, enhanced biosynthesis of hydrogen peroxide, lipid peroxidation and methylglyoxal (MG) in E. sibiricus. Moreover, As-phytotoxicity reduced photosynthetic activities and growth of plants. Results showed that individual and combined treatments, CuNPs (100 mM) as well as soil inoculation of AMF significantly enhanced root growth and shoot growth by declining As content in root tissues and shoot tissues in As polluted soils. E. sibiricus plants treated with CuNPs (100 mM) and/or AMF alleviated As induced phytotoxicity through upregulating the activity of antioxidative enzymes such as catalase (CAT) and superoxide dismutase (SOD) besides the biosynthesis of non-enzymatic antioxidants including phytochelatin (PC) and glutathione (GSH). In brief, supplementation of CuNPs (100 mM) alone or in combination with AMF reduced As uptake and alleviated the As-phytotoxicity in E. sibiricus by inducing stress tolerance mechanism resulting in the improvement of the plant growth parameters.
Keywords: Arsenic toxicity; Nanoparticles; Oxidative stress; Stress markers; Stress mitigation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Arbuscular mycorrhizal fungi and nitric oxide alleviate cadmium phytotoxicity by improving internal detoxification mechanisms of corn plants.Environ Sci Pollut Res Int. 2023 Sep;30(41):93602-93616. doi: 10.1007/s11356-023-28969-w. Epub 2023 Jul 28. Environ Sci Pollut Res Int. 2023. PMID: 37507565
-
Fullerenol nanoparticles and AMF application for optimization of Brassica napus L. resilience to lead toxicity through physio-biochemical and antioxidative modulations.Sci Rep. 2024 Dec 28;14(1):30992. doi: 10.1038/s41598-024-82086-3. Sci Rep. 2024. PMID: 39730765 Free PMC article.
-
Arbuscular mycorrhizal fungi reduce arsenic uptake and improve plant growth in Lens culinaris.PLoS One. 2019 May 16;14(5):e0211441. doi: 10.1371/journal.pone.0211441. eCollection 2019. PLoS One. 2019. PMID: 31095573 Free PMC article.
-
Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization.J Exp Bot. 2002 May;53(372):1351-65. J Exp Bot. 2002. PMID: 11997381 Review.
-
Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses.Plants (Basel). 2023 Aug 29;12(17):3102. doi: 10.3390/plants12173102. Plants (Basel). 2023. PMID: 37687353 Free PMC article. Review.
Cited by
-
Magnesium Oxide Nanoparticles Improved Drought Resilience in L. through Lifting Antioxidant Level, Redox Balancing, and Improving Photosynthetic Efficiency.ACS Omega. 2025 Jul 22;10(30):32813-32828. doi: 10.1021/acsomega.5c00986. eCollection 2025 Aug 5. ACS Omega. 2025. PMID: 40787360 Free PMC article.
-
Evaluation of the molecular mechanism underlying proline metabolic and catabolic pathways and some morpho-physiological traits of tobacco (Nicotiana tabacum L.) plants under arsenic stress.BMC Plant Biol. 2025 Feb 25;25(1):258. doi: 10.1186/s12870-025-06262-x. BMC Plant Biol. 2025. PMID: 40000937 Free PMC article.
References
-
- Mishra RK, Tiwari S, Patel A, Prasad SM. Arsenic contamination, speciation, toxicity and defense strategies in plants. Brazilian J Bot. 2021;44(1):1–10. doi: 10.1007/s40415-020-00694-5. - DOI
-
- Upadhyay MK, Yadav P, Shukla A, Srivastava S. Utilizing the potential of microorganisms for managing arsenic contamination: a feasible and sustainable approach. Front Environ Sci. 2018;6:24. doi: 10.3389/fenvs.2018.00024. - DOI
-
- Alka S, Shahir S, Ibrahim N, Ndejiko MJ, Vo DVN, Manan A. Arsenic removal technologies and future trends: a mini review. J Clean Prod. 2021;278:123805. doi: 10.1016/j.jclepro.2020.123805. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

