Deletion of myeloid HDAC3 promotes efferocytosis to ameliorate retinal ischemic injury
- PMID: 38997746
- PMCID: PMC11241909
- DOI: 10.1186/s12974-024-03159-8
Deletion of myeloid HDAC3 promotes efferocytosis to ameliorate retinal ischemic injury
Abstract
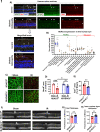
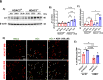
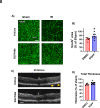
Ischemia-induced retinopathy is a hallmark finding of common visual disorders including diabetic retinopathy (DR) and central retinal artery and vein occlusions. Treatments for ischemic retinopathies fail to improve clinical outcomes and the design of new therapies will depend on understanding the underlying disease mechanisms. Histone deacetylases (HDACs) are an enzyme class that removes acetyl groups from histone and non-histone proteins, thereby regulating gene expression and protein function. HDACs have been implicated in retinal neurovascular injury in preclinical studies in which nonspecific HDAC inhibitors mitigated retinal injury. Histone deacetylase 3 (HDAC3) is a class I histone deacetylase isoform that plays a central role in the macrophage inflammatory response. We recently reported that myeloid cells upregulate HDAC3 in a mouse model of retinal ischemia-reperfusion (IR) injury. However, whether this cellular event is an essential contributor to retinal IR injury is unknown. In this study, we explored the role of myeloid HDAC3 in ischemia-induced retinal neurovascular injury by subjecting myeloid-specific HDAC3 knockout (M-HDAC3 KO) and floxed control mice to retinal IR. The M-HDAC3 KO mice were protected from retinal IR injury as shown by the preservation of inner retinal neurons, vascular integrity, and retinal thickness. Electroretinography confirmed that this neurovascular protection translated to improved retinal function. The retinas of M-HDAC3 KO mice also showed less proliferation and infiltration of myeloid cells after injury. Interestingly, myeloid cells lacking HDAC3 more avidly engulfed apoptotic cells in vitro and after retinal IR injury in vivo compared to wild-type myeloid cells, suggesting that HDAC3 hinders the reparative phagocytosis of dead cells, a process known as efferocytosis. Further mechanistic studies indicated that although HDAC3 KO macrophages upregulate the reparative enzyme arginase 1 (A1) that enhances efferocytosis, the inhibitory effect of HDAC3 on efferocytosis is not solely dependent on A1. Finally, treatment of wild-type mice with the HDAC3 inhibitor RGFP966 ameliorated the retinal neurodegeneration and thinning caused by IR injury. Collectively, our data show that HDAC3 deletion enhances macrophage-mediated efferocytosis and protects against retinal IR injury, suggesting that inhibiting myeloid HDAC3 holds promise as a novel therapeutic strategy for preserving retinal integrity after ischemic insult.
Keywords: Efferocytosis; Histone deacetylase 3; Macrophages; Microglia; Retinal ischemia-reperfusion injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Shosha E, Shahror RA, Morris CA, Xu Z, Lucas R, McGee-Lawrence ME, et al. The arginase 1/ornithine decarboxylase pathway suppresses hdac3 to ameliorate the myeloid cell inflammatory response: implications for retinal ischemic injury. Cell Death Dis. 2023;14:621. doi: 10.1038/s41419-023-06147-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

