The integrin receptor beta7 subunit mediates airway remodeling and hyperresponsiveness in allergen exposed mice
- PMID: 38997751
- PMCID: PMC11241790
- DOI: 10.1186/s12931-024-02899-8
The integrin receptor beta7 subunit mediates airway remodeling and hyperresponsiveness in allergen exposed mice
Abstract
Background: Fibroblast differentiation to a myofibroblast phenotype is a feature of airway remodeling in asthma. Lung fibroblasts express the integrin receptor α4β7 and fibronectin induces myofibroblast differentiation via this receptor.
Objectives: To investigate the role of the β7 integrin receptor subunit and α4β7 integrin complex in airway remodeling and airway hyperresponsiveness (AHR) in a murine model of chronic allergen exposure.
Methods: C57BL/6 wild type (WT) and β7 integrin null mice (β7 -/-) were sensitized (days 1,10) and challenged with ovalbumin (OVA) three times a week for one or 4 weeks. Similar experiments were performed with WT mice in the presence or absence of α4β7 blocking antibodies. Bronchoalveolar (BAL) cell counts, AHR, histological evaluation, soluble collagen content, Transforming growth factor-β (TGFβ) and Interleukin-13 (IL13) were measured. Phenotype of fibroblasts cultured from WT and β7 -/- saline (SAL) and OVA treated mice was evaluated.
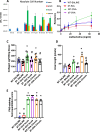
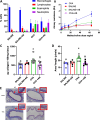
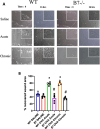
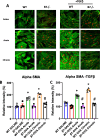
Results: Eosinophil numbers were similar in WT vs β7-/- mice. Prolonged OVA exposure in β7-/- mice was associated with reduced AHR, lung collagen content, peribronchial smooth muscle, lung tissue TGFβ and IL13 expression as compared to WT. Similar findings were observed in WT mice treated with α4β7 blocking antibodies. Fibroblast migration was enhanced in response to OVA in WT but not β7 -/- fibroblasts. α-SMA and fibronectin expression were reduced in β7-/- fibroblasts relative to WT.
Conclusions: The β7 integrin subunit and the α4β7 integrin complex modulate AHR and airway remodeling in a murine model of allergen exposure. This effect is, at least in part, explained by inhibition of fibroblast activation and is independent of eosinophilic inflammation.
Keywords: Airway-hyperresponsiveness; Asthma; Fibroblast; Remodeling; α4β7 integrin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Airway hyperresponsiveness is associated with airway remodeling but not inflammation in aging Cav1-/- mice.Respir Res. 2013 Oct 21;14(1):110. doi: 10.1186/1465-9921-14-110. Respir Res. 2013. PMID: 24138138 Free PMC article.
-
Inhibition of allergen-induced airway remodeling in Smad 3-deficient mice.J Immunol. 2007 Jun 1;178(11):7310-6. doi: 10.4049/jimmunol.178.11.7310. J Immunol. 2007. PMID: 17513781
-
Osteopontin induces airway remodeling and lung fibroblast activation in a murine model of asthma.Am J Respir Cell Mol Biol. 2009 Sep;41(3):290-6. doi: 10.1165/rcmb.2008-0307OC. Epub 2009 Jan 16. Am J Respir Cell Mol Biol. 2009. PMID: 19151319
-
Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13.Respir Res. 2010 Nov 1;11(1):154. doi: 10.1186/1465-9921-11-154. Respir Res. 2010. PMID: 21040544 Free PMC article.
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

