Identification of Two Linear Epitopes on MGF_110-13L Protein of African Swine Fever Virus with Monoclonal Antibodies
- PMID: 38998063
- PMCID: PMC11240426
- DOI: 10.3390/ani14131951
Identification of Two Linear Epitopes on MGF_110-13L Protein of African Swine Fever Virus with Monoclonal Antibodies
Abstract
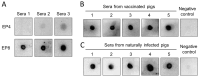
African swine fever caused by African swine fever virus (ASFV) is an acute, highly contagious swine disease with high mortality. To facilitate effective vaccine development and find more serodiagnostic targets, fully exploring the ASFV antigenic proteins is urgently needed. In this study, the MGF_110-13L was identified as an immunodominant antigen among the seven transmembrane proteins. The main outer-membrane domain of MGF_110-13L was expressed and purified. Two monoclonal antibodies (mAbs; 8C3, and 10E4) against MGF_110-13L were generated. The epitopes of two mAbs were preliminary mapped with the peptide fusion proteins after probing with mAbs by enzyme-linked immunosorbent assay (ELISA) and Western blot. And the two target epitopes were fine-mapped using further truncated peptide fusion protein strategy. Finally, the core sequences of mAbs 8C3 and 10E4 were identified as 48WDCQDGICKNKITESRFIDS67, and 122GDHQQLSIKQ131, respectively. The peptides of epitopes were synthesized and probed with ASFV antibody positive pig sera by a dot blot assay, and the results showed that epitope 10E4 was an antigenic epitope. The epitope 10E4 peptide was further evaluated as a potential antigen for detecting ASFV antibodies. To our knowledge, this is the first report of antigenic epitope information on the antigenic MGF_110-13L protein of ASFV.
Keywords: African swine fever virus; MGF_110-13L protein; epitope; monoclonal antibody.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Penrith M.-L. History of ‘swine fever’ in Southern Africa. J. S. Afr. Vet. Assoc. 2013;84:a1106. doi: 10.4102/jsava.v84i1.1106. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

