Effects of Luteolin in an In Vitro Model of Porcine Intestinal Infections
- PMID: 38998064
- PMCID: PMC11240391
- DOI: 10.3390/ani14131952
Effects of Luteolin in an In Vitro Model of Porcine Intestinal Infections
Abstract
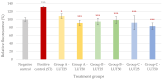
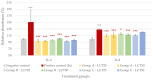
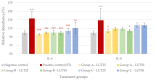
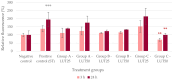
Intestinal infections caused by Escherichia coli and Salmonella enterica pose a huge economic burden on the swine industry that is exacerbated by the development of antimicrobial resistance in these pathogens, thus raising the need for alternative prevention and treatment methods. Our aim was to test the beneficial effects of the flavonoid luteolin in an in vitro model of porcine intestinal infections. We infected the porcine intestinal epithelial cell line IPEC-J2 with E. coli and S. enterica subsp. enterica serovar Typhimurium (106 CFU/mL) with or without previous, concurrent, or subsequent treatment with luteolin (25 or 50 µg/mL), and measured the changes in the reactive oxygen species and interleukin-6 and -8 levels of cells. We also tested the ability of luteolin to inhibit the adhesion of bacteria to the cell layer, and to counteract the barrier integrity damage caused by the pathogens. Luteolin was able to alleviate oxidative stress, inflammation, and barrier integrity damage, but it could not inhibit the adhesion of bacteria to IPEC-J2 cells. Luteolin is a promising candidate to be used in intestinal infections of pigs, however, further studies are needed to confirm its efficacy. The use of luteolin in the future could ultimately lead to a reduced need for antibiotics in pig production.
Keywords: AMR; Escherichia coli; IPEC-J2; Salmonella enterica; bacterial infections; flavonoids; intestines; luteolin; pigs; porcine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

