Chlorophyllin Supplementation of Medicated or Unmedicated Swine Diets Impact on Fecal Escherichia coli and Enterococci
- PMID: 38998066
- PMCID: PMC11240447
- DOI: 10.3390/ani14131955
Chlorophyllin Supplementation of Medicated or Unmedicated Swine Diets Impact on Fecal Escherichia coli and Enterococci
Abstract
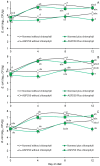
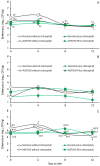
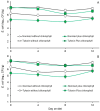
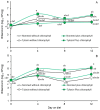
Considering that certain catabolic products of anaerobic chlorophyll degradation inhibit efflux pump activity, this study was conducted to test if feeding pigs a water-soluble chlorophyllin product could affect the antibiotic resistance profiles of select wild-type populations of fecal bacteria. Trial 1 evaluated the effects of chlorophyllin supplementation (300 mg/meal) on fecal E. coli and enterococcal populations in pigs fed twice daily diets supplemented without or with ASP 250 (containing chlortetracycline, sulfamethazine and penicillin at 100, 100 and 50 g/ton, respectively). Trial 2, conducted similarly, evaluated chlorophyllin supplementation in pigs fed diets supplemented with or without 100 g tylosin/ton. Each trial lasted 12 days, and fecal samples were collected and selectively cultured at 4-day intervals to enumerate the total numbers of E. coli and enterococci as well as populations of these bacteria phenotypically capable of growing in the presence of the fed antibiotics. Performance results from both studies revealed no adverse effect (p > 0.05) of chlorophyllin, antibiotic or their combined supplementation on average daily feed intake or average daily gain, although the daily fed intake tended to be lower (p = 0.053) for pigs fed diets supplemented with tylosin than those fed diets without tylosin. The results from trial 1 showed that the ASP 250-medicated diets, whether without or with chlorophyllin supplementation, supported higher (p < 0.05) fecal E. coli populations than the non-medicated diets. Enterococcal populations, however, were lower, albeit marginally and not necessarily significantly, in feces from pigs fed the ASP 250-medicated diet than those fed the non-medicated diet. Results from trial 2 likewise revealed an increase (p < 0.05) in E. coli and, to a lesser extent, enterococcal populations in feces collected from pigs fed the tylosin-medicated diet compared with those fed the non-medicated diet. Evidence indicated that the E. coli and enterococcal populations in trial 1 were generally insensitive to penicillin or chlortetracycline, as there were no differences between populations recovered without or with antibiotic selection. Conversely, a treatment x day of treatment interaction observed in trial 2 (p < 0.05) provided evidence, albeit slight, of an enrichment of tylosin-insensitive enterococci in feces from the pigs fed the tylosin-medicated but not the non-medicated diet. Under the conditions of the present study, it is unlikely that chlorophyllin-derived efflux pump inhibitors potentially present in the chlorophyllin-fed pigs were able to enhance the efficacy of the available antibiotics. However, further research specifically designed to optimize chlorophyll administration could potentially lead to practical applications for the swine industry.
Keywords: antimicrobial resistance; chlorophyll metabolites; efflux pump inhibitors; medicated feed; zoonotic pathogens.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Wu F., Tokach M.D., DeRouchey J.M., Dritz S.S., Woodworth J.C., Goodband R.D., Chitakasempornkul K., Bello N.M., Capps K., Remfry S., et al. Effects of tylosin administration routes on the prevalence of antimicrobial resistance among fecal enterococci of finishing swine. Foodborne Pathog. Dis. 2019;16:309–316. doi: 10.1089/fpd.2018.2551. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

