Structural and Release Properties of Combined Curcumin Controlled-Release Tablets Formulated with Chitosan/Sodium Alginate/HPMC
- PMID: 38998528
- PMCID: PMC11241607
- DOI: 10.3390/foods13132022
Structural and Release Properties of Combined Curcumin Controlled-Release Tablets Formulated with Chitosan/Sodium Alginate/HPMC
Abstract
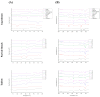
Controlled-release tablets offer several benefits, such as controlled release, odor masking, ease of use, stability, extended shelf life, and reduced production costs. This study developed combined curcumin controlled-release tablets (CCCTs) to increase the bioavailability of curcumin with hydroxypropyl methylcellulose (HPMC), chitosan, and sodium alginate. The hardness of the CCCTs was 5.63-1.98 kgf, friability was 0.00-1.22%, and disintegration time was 0.00-401.25 min. Differential scanning calorimetry and Fourier-transform infrared spectroscopy indicated a high compatibility between the excipients and curcumin. CCCTs with chitosan formed a gel structure, impeded disintegration, and reduced the release rate to 72.5% in simulated gastric fluid. In simulated intestinal fluid, CCCT with the HPMC-sodium alginate group formed a polyelectrolyte membrane hydrogel to prolong release from 6 to 12 h. This study developed various CCCT formulations that can be delivered through the gastric or intestinal tracts, using chitosan and HPMC-sodium alginate as excipients, respectively. CCCT can be used as a reference strategy for controlled-release curcumin delivery in the functional and healthcare supplement development.
Keywords: combined controlled-release tablet; curcumin; simulated gastrointestinal tract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Yuan K.C., Chiang Y.C., Li P.H., Chiang P.Y. Physicochemical and release properties of anthocyanin gastric floating tablets colloidized with κ-carrageenan/metal ions. Food Hydrocoll. 2024;150:109674. doi: 10.1016/j.foodhyd.2023.109674. - DOI
-
- López-Córdoba A., Matera S., Deladino L., Hoya A., Navarro A., Martino M. Compressed tablets based on mineral-functionalized starch and co-crystallized sucrose with natural antioxidants. J. Food Eng. 2015;146:234–242. doi: 10.1016/j.jfoodeng.2014.09.019. - DOI
LinkOut - more resources
Full Text Sources

