Preliminary Report of Nationwide COVID-19 Vaccine Compensation in Taiwan
- PMID: 38998785
- PMCID: PMC11241583
- DOI: 10.3390/healthcare12131250
Preliminary Report of Nationwide COVID-19 Vaccine Compensation in Taiwan
Abstract
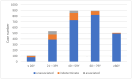
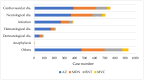
The potential adverse effects of coronavirus disease 2019 (COVID-19) vaccinations raise public concerns. Data from Taiwan's Vaccine Injury Compensation Program (VICP) can provide valuable insights. This study analyzed the preliminary application data for COVID-19 vaccine compensation in Taiwan's VICP, focusing on applicants receiving vaccines between March 2021 and June 2022. Among the 2941 adverse events, 113 cases (3.8%) were deemed causally associated with vaccination, 313 (10.6%) were indeterminate, and 2515 (85.5%) had no causal association. Nearly half (47.6%) of the applicants were over 60 years old, and 76.6% had a history of pre-existing chronic diseases. Among the 426 vaccine-associated or indeterminate cases, the most common causes were hematological diseases and thrombosis. There were 920 mortality cases reported, and 97.4% were unassociated with vaccination. Only five deaths were judged to be associated with the COVID-19 vaccination, all involving the adenovirus vector vaccine and thrombosis with thrombocytopenia syndrome. In conclusion, most compensation applications were not causally linked to vaccination. Compared to other countries, the number of applications in Taiwan's VICP is relatively high. These findings may indicate a need to adjust the application requirements for compensation in Taiwan's program.
Keywords: Vaccine Injury Compensation Program; coronavirus disease 2019 vaccine; vaccine adverse events.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Spectrum and Incidence of Adverse Reactions Post Immunization in the Taiwanese Population (2014-2019): An Analysis Using the National Vaccine Injury Compensation Program.Vaccines (Basel). 2024 Oct 3;12(10):1133. doi: 10.3390/vaccines12101133. Vaccines (Basel). 2024. PMID: 39460300 Free PMC article.
-
COVID-19 Vaccine Injury Compensation Program: Lessons Learned From a Review of 10 Implementing Countries.J Korean Med Sci. 2024 Apr 8;39(13):e121. doi: 10.3346/jkms.2024.39.e121. J Korean Med Sci. 2024. PMID: 38599598 Free PMC article. Review.
-
Incidence and Nature of Short-Term Adverse Events following COVID-19 Second Boosters: Insights from Taiwan's Universal Vaccination Strategy.Vaccines (Basel). 2024 Jan 31;12(2):149. doi: 10.3390/vaccines12020149. Vaccines (Basel). 2024. PMID: 38400133 Free PMC article.
-
Current situation of vaccine injury compensation program and a future perspective in light of COVID-19 and emerging viral diseases.F1000Res. 2021 Jul 26;10:652. doi: 10.12688/f1000research.51160.2. eCollection 2021. F1000Res. 2021. PMID: 35035888 Free PMC article. Review.
-
Performance of the United States Vaccine Injury Compensation Program (VICP): 1988-2019.Vaccine. 2020 Feb 24;38(9):2136-2143. doi: 10.1016/j.vaccine.2020.01.042. Epub 2020 Jan 22. Vaccine. 2020. PMID: 31982259 Free PMC article. Review.
References
-
- Food and Drug Administration, Ministry of Health and Welfare TFDA Granted Emergency Use Authorization (EUA) for Four COVID-19 Vaccines in Taiwan. [(accessed on 30 March 2024)];2021 Available online: https://www.mohw.gov.tw/cp-115-64023-2.html.
-
- Goddard K., Lewis N., Fireman B., Weintraub E., Shimabukuro T., Zerbo O., Boyce T.G., Oster M.E., Hanson K.E., Donahue J.G., et al. Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination. Vaccine. 2022;40:5153–5159. doi: 10.1016/j.vaccine.2022.07.007. - DOI - PMC - PubMed
-
- Rosenblum H.G., Gee J., Liu R., Marquez P.L., Zhang B., Strid P., Abara W.E., McNeil M.M., Myers T.R., Hause A.M., et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect. Dis. 2022;22:802–812. doi: 10.1016/s1473-3099(22)00054-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

