Ionic Liquid-Based Immunization Patch for the Transdermal Delivery of Antigens
- PMID: 38998948
- PMCID: PMC11243093
- DOI: 10.3390/molecules29132995
Ionic Liquid-Based Immunization Patch for the Transdermal Delivery of Antigens
Abstract
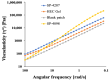
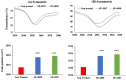
Herein, we report a transdermal patch prepared using an ionic liquid-based solid in oil (IL-S/O) nanodispersion and a pressure-sensitive adhesive (PSA) to deliver the macromolecular antigenic protein, ovalbumin (OVA). The IL-S/O nanodispersion and a PSA were first mixed at an equal weight ratio, then coated onto a release liner, and covered with a support film. To evaluate the effect of the PSA, three types of PSAs, DURO-TAK 87-4098, DURO-TAK 87-4287, and DURO-TAK 87-235A, were used to obtain the corresponding IL-S/O patches SP-4098, SP-4287, and SP-235A, respectively. The prepared IL-S/O patches were characterized for surface morphology, viscoelasticity, and moisture content. In vitro skin penetration and in vivo immunization studies of the IL-S/O patches were performed using Yucatan micropig skin and the C57BL/6NJc1 mice model, respectively. The SP-4098 and SP-4287 delivered 5.49-fold and 5.47-fold higher amounts of drug compared with the aqueous formulation. Although both patches delivered a similar amount of drug, SP-4287 was not detached fully from the release liner after 30 days, indicating low stability. Mice immunized with the OVA-containing SP-4098 produced a 10-fold increase in anti-OVA IgG compared with those treated with an aqueous formulation. These findings suggested that the IL-S/O patch may be a good platform for the transdermal delivery of antigen molecules.
Keywords: IL-S/O patch; immunization; ionic liquid; pressure-sensitive adhesive; transdermal drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- World Health Ortanization . Immunization Agenda 2030. WHO; Geneva, Switzerland: 2022. pp. 1–58.
-
- Zhu W., Wei T., Xu Y., Jin Q., Chao Y., Lu J., Xu J., Zhu J., Yan X., Chen M., et al. Non-Invasive Transdermal Delivery of Biomacromolecules with Fluorocarbon-Modified Chitosan for Melanoma Immunotherapy and Viral Vaccines. Nat. Commun. 2024;15:820. doi: 10.1038/s41467-024-45158-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

