Preparation of Quaternary Ammonium Separation Material based on Coupling Agent Chloromethyl Trimethoxysilane (KH-150) and Its Adsorption and Separation Properties in Studies of Th(IV)
- PMID: 38998982
- PMCID: PMC11243607
- DOI: 10.3390/molecules29133031
Preparation of Quaternary Ammonium Separation Material based on Coupling Agent Chloromethyl Trimethoxysilane (KH-150) and Its Adsorption and Separation Properties in Studies of Th(IV)
Abstract
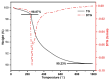
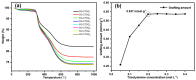
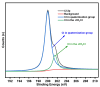
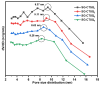
In this research, the authors studied the synthesis of a silicon-based quaternary ammonium material based on the coupling agent chloromethyl trimethoxysilane (KH-150) as well as its adsorption and separation properties for Th(IV). Using FTIR and NMR methods, the silicon-based materials before and after grafting were characterized to determine the spatial structure of functional groups in the silicon-based quaternary ammonium material SG-CTSQ. Based on this, the functional group grafting amount (0.537 mmol·g-1) and quaternization rate (83.6%) of the material were accurately calculated using TGA weight loss and XPS. In the adsorption experiment, the four materials with different grafting amounts showed different degrees of variation in their adsorption of Th(IV) with changes in HNO3 concentration and NO3- concentration but all exhibited a tendency toward anion exchange. The thermodynamic and kinetic experimental results demonstrated that materials with low grafting amounts (SG-CTSQ1 and SG-CTSQ2) tended to physical adsorption of Th(IV), while the other two tended toward chemical adsorption. The adsorption mechanism experiment further proved that the functional groups achieve the adsorption of Th(IV) through an anion-exchange reaction. Chromatographic column separation experiments showed that SG-CTSQ has a good performance in U-Th separation, with a decontamination factor for uranium in Th(IV) of up to 385.1, and a uranium removal rate that can reach 99.75%.
Keywords: Th(IV); adsorption; coupling agent; quaternary ammonium; separation material.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures













References
-
- Galahom A. Minimization of the fission product waste by using thorium based fuel instead of uranium dioxide. Nucl. Eng. Des. 2017;314:165–172. doi: 10.1016/j.nucengdes.2017.01.024. - DOI
-
- Leotlela M., Hadebe N., Petr I. Prediction of dose rates around the interim spent fuel storage facility. Radiat. Phys. Chem. 2020;11:197–211. doi: 10.1016/j.radphyschem.2020.109171. - DOI
-
- Akbari M., Tonkaboni S., Khanchi A. Thorium Recovery from Choghart Mining Waste by Beneficiation Processes. JOM. 2023;75:1045–1058. doi: 10.1007/s11837-023-05706-9. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

