Stable Radical Isoporphyrin Copolymer Prepared with Di(phenylphosphane)
- PMID: 38999012
- PMCID: PMC11243264
- DOI: 10.3390/molecules29133056
Stable Radical Isoporphyrin Copolymer Prepared with Di(phenylphosphane)
Abstract
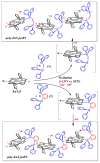
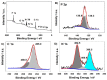
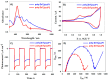
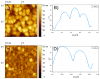
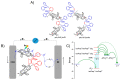
Two diphosphanes with variable-length ligands tested as nucleophiles to prepare isoporphyrin copolymers in the presence of ditolylporphyrin of zinc (ZnT2P) prevented the oxidation of the diphosphine ligand. This paper demonstrates the power of this approach and describes the photoelectrocatalytic properties. The obtained copolymers were characterized by UV-vis spectroscopy, X-ray photoelectron spectroscopy, atomic force micrograph (AFM), EQCM (Electrochemical Quartz Cristal Microbalance) and electrochemistry. Their impedance properties (EIS) were studied and their photovoltaic performances were also investigated by photocurrent transient measurements under visible light irradiation.
Keywords: EPR spectroscopy; EQCM; electrochemistry; phosphonium; photocurrent; porphyrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Evans B., Smith K.M. Novel meso-substitution reactions of zinc (II) octaethylporphyrin. Tetrahedron Lett. 1977;18:3079–3082. doi: 10.1016/S0040-4039(01)83163-4. - DOI
-
- Smith K.M., Barnett G.H., Evans B., Martynenko Z. Novel meso-substitution reactions of metalloporphyrins. J. Am. Chem. Soc. 1979;101:5953–5961. doi: 10.1021/ja00514a015. - DOI
-
- Padilla A.G., Wu S.-M., Shine H.J. Reaction of zinc tetraphenylporphyrin cation radical perchlorate with pyridine. J. Chem. Soc. Chem. Commun. 1976;7:236–237. doi: 10.1039/c39760000236. - DOI
-
- Shine H.J., Padilla A.G., Wu S.-M. Ion radicals. 45. Reactions of zinc tetraphenylporphyrin cation radical perchlorate with nucleophiles. J. Org. Chem. 1979;44:4069–4075. doi: 10.1021/jo01337a010. - DOI
-
- Malek A., Latos-Grazynski L., Bartczak T.J., Zadlo A. Reactions of the iron(III) tetraphenylporphyrin.pi. cation radical with triphenylphosphine and the nitrite anion. Formation of β-substituted iron(III) porphyrins. Inorg. Chem. 1991;30:3222–3230. doi: 10.1021/ic00016a021. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

