Isovaleryl Sucrose Esters from Atractylodes japonica and Their Cytotoxic Activity
- PMID: 38999021
- PMCID: PMC11243297
- DOI: 10.3390/molecules29133069
Isovaleryl Sucrose Esters from Atractylodes japonica and Their Cytotoxic Activity
Abstract
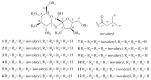
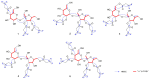
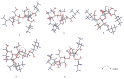
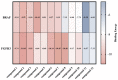
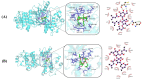
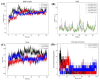
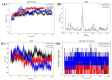
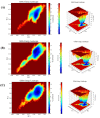
Cancer represents one of the most significant health challenges currently facing humanity, and plant-derived antitumour drugs represent a prominent class of anticancer medications in clinical practice. Isovaleryl sucrose esters, which are natural constituents, have been identified as having potential antitumour effects. However, the mechanism of action remains unclear. In this study, 12 isovaleryl sucrose ester components, including five new (1-5) and seven known compounds (6-12), were isolated from the roots of Atractylodes japonica. The structures of the compounds were elucidated using 1D and 2D-NMR spectroscopy, complemented by HR-ESI-MS mass spectrometry. The cytotoxic activities of all the compounds against human colon cancer cells (HCT-116) and human lung adenocarcinoma cells (A549) were also evaluated using the CCK8 assay. The results demonstrated that compounds 2, 4, and 6 were moderately inhibitory to HCT-116 cells, with IC50 values of 7.49 ± 0.48, 9.03 ± 0.21, and 13.49 ± 1.45 μM, respectively. Compounds 1 and 6 were moderately inhibitory to A549, with IC50 values of 8.36 ± 0.77 and 7.10 ± 0.52 μM, respectively. Molecular docking revealed that compounds 1-9 exhibited a stronger affinity for FGFR3 and BRAF, with binding energies below -7 kcal/mol. Compound 2 exhibited the lowest binding energy of -10.63 kcal/mol to FGFR3. We screened the compounds with lower binding energies, and the protein-ligand complexes already obtained after molecular docking were subjected to exhaustive molecular dynamics simulation experiments, which simulated the dynamic behaviour of the molecules in close proximity to the actual biological environment, thus providing a deeper understanding of their functions and interaction mechanisms. The present study provides a reference for the development and use of iso-valeryl sucrose esters in the antitumour field.
Keywords: cytotoxic activity; isovaleryl sucrose esters; molecular docking; molecular dynamics simulation; phytochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Sun J., Zhao J., Jiang F., Wang L., Xiao Q., Han F., Chen J., Yuan S., Wei J., Larsson S.C., et al. Identification of novel protein biomarkers and drug targets for colorectal cancer by integrating human plasma proteome with genome. Genome Med. 2023;15:75. doi: 10.1186/s13073-023-01229-9. - DOI - PMC - PubMed
-
- Zhu Y., Wang A., Zhang S., Kim J., Xia J., Zhang F., Wang D., Wang Q., Wang J. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J. Adv. Res. 2023;49:159–173. doi: 10.1016/j.jare.2022.09.007. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- Grant Number: [2018] No. 284/Qi-Huang Scholar of National Traditional Chinese Medicine Leading Talents Support Program
- Grant Number: [2019] No. 5/Heilongjiang Touyan Innovation Team Program (Grant Number: [2019] No. 5).
- Grant Number: [2022] No. 76/The Seventh Batch of National Famous Old Traditional Chinese Medicine Experts Ex-perience Heritage Construction Program of National Administration of TCM, (Grant Number: [2022] No. 76),
- Grant Number: [2022] No. 75/National Famous Old Traditional Chinese Medicine Experts Inheritance Studio Con-struction Program of National Administration of TCM (Grant Number: [2022] No. 75),
- Grant Number: [2018] No. 284/Qi-Huang Scholar of National Traditional Chinese Medicine Leading Talents Support Program (Grant Number: [2018] No. 284),
LinkOut - more resources
Full Text Sources
Research Materials

