Long-Term Persistence Rate of Secukinumab in Psoriatic Patients: A Six-Year Multicenter, Real-World Experience, Retrospective Study
- PMID: 38999429
- PMCID: PMC11242555
- DOI: 10.3390/jcm13133864
Long-Term Persistence Rate of Secukinumab in Psoriatic Patients: A Six-Year Multicenter, Real-World Experience, Retrospective Study
Abstract
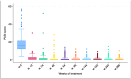
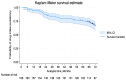
Background: Psoriatic disease, a chronic immune-mediated systemic inflammatory condition, significantly impairs patients' quality of life. The advent of highly targeted biological therapies has transformed treatment strategies, emphasizing the importance of selecting the most effective and cost-efficient option. Secukinumab, an IL-17A inhibitor, has demonstrated efficacy and safety in treating moderate-to-severe plaque psoriasis (PsO). However, long-term real-world data on its effectiveness and persistence rate are limited. Methods: This retrospective study, conducted across eight Italian dermatology centers, aimed to evaluate the 6-year persistence rate and effectiveness of secukinumab in patients with PsO. Additionally, the study investigated the onset of psoriatic arthritis during treatment. Results: Overall, 166 adult patients were analyzed. Their median age was 53.9 years. The mean BMI was 26.5. Of the 166 patients, 64 were bio-experienced while 102 were bio-naïve. A progressive reduction in PsO severity measured by PASI scores over 6 years of treatment was revealed: the PASI score decreased from a baseline value of 18.1 (±9.1) to 0.7 (±1.6) after 6 years of follow-up. Adverse events, including mucocutaneous fungal infections and cardiovascular disturbances, were reported in 19.9% of patients. The persistence rate was 86.8% at 24 months, decreasing to 66.4% at 72 months. Psoriatic arthritis onset during treatment was observed in 15 (9.0%) of patients. Conclusions: This study highlights the sustained effectiveness and favorable safety profile of secukinumab over 6 years, providing valuable real-world evidence. Understanding the long-term persistence rate and predictors of discontinuation could help clinicians optimize treatment decisions and improve patient outcomes in PsO management. We found that the absence of scalp PsO, no involvement of the genital area and normal weight were the best factors of persistence in secukinumab treatment in the long term.
Keywords: biologics; comorbidities; persistence rate; psoriasis; secukinumab.
Conflict of interest statement
M.G. has acted as a speaker and/or consultant for AbbVie, Almirall, Eli-Lilly, Janssen-Cilag, LeoPharma, Novartis, and Sanofi outside the submitted work; M.T. has acted as a speaker and/or consultant for AbbVie, Almirall, Eli-Lilly, Janssen-Cilag, LeoPharma, Novartis, and Sanofi outside the submitted work; G.C. has received consulting fees, honoraria, and support for attending meetings from Abbvie, Lilly, Almirall, Janssen, uCB, Novartis and Leopharma; M.B. served as a speaker or consultant for Abbvie, Amgen, Almirall, Eli Lilly, Novartis, Janssen, and UCB; R.B. has received support for attending meetings and/or travel for AbbVie, Amgen, Leo Pharma, Lilly, Novartis, and Sanofi; FR has acted as a speaker and consultant for Sanofi, Abbvie, and Novartis; C.D.S. served as a speaker or consultant for Abbvie, Almirall, Boehringer Ingelheim, Eli Lilly, Leopharma, Janssen, Novartis, Pfizer, Pierre Fabre, Sanofi, and UCB Pharma; G.R. has received support for attending meetings and/or travel for AbbVie, Amgen, Lilly, and Novartis; E.C.C. served as a speaker or consultant for Abbvie, Almirall, Eli Lilly, Novartis, Janssen, and UCB; L.A. is part of the advisory board and a consultant for Abvvie, Almirall, Amgen, BMS, Ely-Lilly, Leopharma, Janssen, Novartis, Sanofi, and UCB; A.C. has served as an advisory board member and consultant and has received fees and speaker’s honoraria or has participated in clinical trials for AbbVie, Almirall, Bristol Myers Squibb, Leo Pharma, Lilly, Janssen, Novartis, Pfizer, and Sanofi Genzyme. All other authors declare no conflicts of interest.
Figures
References
-
- Augustin M., Sator P.G., von Kiedrowski R., Conrad C., Rigopoulos D., Romanelli M., Ghislain P.D., Torres T., Ioannides D., Aassi M., et al. Secukinumab demonstrated sustained retention, effectiveness and safety in a real-world setting in patients with moderate-to-severe plaque psoriasis, long-term results from an interim analysis of the SERENA study. J. Eur. Acad. Dermatol. Venereol. 2022;36:1796–1804. doi: 10.1111/jdv.18329. - DOI - PubMed
-
- Gargiulo L., Ibba L., Malagoli P., Balato A., Bardazzi F., Burlando M., Carrera C.G., Damiani G., Dapavo P., Dini V., et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for moderate-to-severe plaque psoriasis, a retrospective multicenter real-world experience on 5932 treatment courses—IL PSO (Italian landscape psoriasis) Front. Immunol. 2024;14:1341708. doi: 10.3389/fimmu.2023.1341708. - DOI - PMC - PubMed
-
- Tada Y., Morita A., Yamanaka K., Kono M., Imafuku S., Okubo Y., Yamazaki F., Kawamura T., Itakura A., Ohtsuki M. Real-world retention rates and effectiveness of secukinumab in psoriasis, Results from a multicenter cohort study (RAILWAY) J. Dermatol. 2023;50:1415–1426. doi: 10.1111/1346-8138.16926. - DOI - PubMed
-
- Asawanonda P., Pattamadilok B., Chularojanamontri L., Chuamanochan M., Choonhakarn C., Chakkavittumrong P., Sangob N., Rajatanavin N. Real-world experience of secukinumab in moderate to severe psoriasis patients in Thailand, Characteristics, effectiveness, and safety. Dermatol. Ther. 2022;35:e15958. doi: 10.1111/dth.15958. - DOI - PMC - PubMed
-
- Foley P., Manuelpillai N., Dolianitis C., Cains G.D., Mate E., Tronnberg R., Baker C. Secukinumab treatment demonstrated high drug survival and sustained effectiveness in patients with severe chronic plaque psoriasis: 21-month analysis in Australian routine clinical practice (SUSTAIN study) Australas. J. Dermatol. 2022;63:303–311. doi: 10.1111/ajd.13895. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

