Final Results from the First European Real-World Experience on Lusutrombopag Treatment in Cirrhotic Patients with Severe Thrombocytopenia: Insights from the REAl-World Lusutrombopag Treatment in ITalY Study
- PMID: 38999529
- PMCID: PMC11242055
- DOI: 10.3390/jcm13133965
Final Results from the First European Real-World Experience on Lusutrombopag Treatment in Cirrhotic Patients with Severe Thrombocytopenia: Insights from the REAl-World Lusutrombopag Treatment in ITalY Study
Abstract
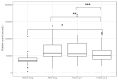
Background and aims: Management of severe thrombocytopenia poses significant challenges in patients with chronic liver disease. Here, we aimed to evaluate the first real-world European post-marketing cohort of cirrhotic patients treated with lusutrombopag, a thrombopoietin receptor agonist, verifying the efficacy and safety of the drug. Methods: In the REAl-world Lusutrombopag treatment in ITalY (REALITY) study, we collected data from consecutive cirrhotic patients treated with lusutrombopag in 19 Italian hepatology centers, mostly joined to the "Club Epatologi Ospedalieri" (CLEO). Primary and secondary efficacy endpoints were the ability of lusutrombopag to avoid platelet transfusions and to raise the platelet count to ≥50,000/μL, respectively. Treatment-associated adverse events were also collected. Results: A total of 66 patients and 73 cycles of treatment were included in the study, since 5 patients received multiple doses of lusutrombopag over time for different invasive procedures. Fourteen patients (19%) had a history of portal vein thrombosis (PVT). Lusutrombopag determined a significant increase in platelet count [from 37,000 (33,000-44,000/μL) to 58,000 (49,000-82,000), p < 0.001]. The primary endpoint was met in 84% of patients and the secondary endpoint in 74% of patients. Baseline platelet count was the only independent factor associated with response in multivariate logistic regression analysis (OR for any 1000 uL of 1.13, CI95% 1.04-1.26, p 0.01), with a good discrimination power (AUROC: 0.78). Notably, a baseline platelet count ≤ 29,000/μL was identified as the threshold for identifying patients unlikely to respond to the drug (sensitivity of 91%). Finally, de novo PVT was observed in four patients (5%), none of whom had undergone repeated treatment, and no other safety or hemorrhagic events were recorded in the entire population analyzed. Conclusions: In this first European real-world series, lusutrombopag demonstrated efficacy and safety consistent with the results of registrational studies. According to our results, patients with baseline platelet counts ≤29,000/μL are unlikely to respond to the drug.
Keywords: lusutrombopag; portal vein thrombosis (PVT); severe thrombocytopenia; thrombopoietin receptor agonists (TPO-RA).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Gallo P., Terracciani F., Di Pasquale G., Esposito M., Picardi A., Vespasiani-Gentilucci U. Thrombocytopenia in chronic liver disease: Physiopathology and new therapeutic strategies before invasive procedures. World J. Gastroenterol. 2022;28:4061–4074. doi: 10.3748/wjg.v28.i30.4061. - DOI - PMC - PubMed
-
- Hidaka H., Kurosaki M., Tanaka H., Kudo M., Abiru S., Igura T., Ishikawa T., Seike M., Katsube T., Ochiai T., et al. Lusutrombopag Reduces Need for Platelet Transfusion in Patients with Thrombocytopenia Undergoing Invasive Procedures. Clin. Gastroenterol. Hepatol. 2019;17:1192–1200. doi: 10.1016/j.cgh.2018.11.047. - DOI - PubMed
-
- Peck-Radosavljevic M., Simon K., Iacobellis A., Hassanein T., Kayali Z., Tran A., Makara M., Ben Ari Z., Braun M., Mitrut P., et al. Lusutrombopag for the Treatment of Thrombocytopenia in Patients with Chronic Liver Disease Undergoing Invasive Procedures (L-PLUS 2) Hepatology. 2019;70:1336–1348. doi: 10.1002/hep.30561. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

