Exploring Aluminum Tolerance Mechanisms in Plants with Reference to Rice and Arabidopsis: A Comprehensive Review of Genetic, Metabolic, and Physiological Adaptations in Acidic Soils
- PMID: 38999600
- PMCID: PMC11243567
- DOI: 10.3390/plants13131760
Exploring Aluminum Tolerance Mechanisms in Plants with Reference to Rice and Arabidopsis: A Comprehensive Review of Genetic, Metabolic, and Physiological Adaptations in Acidic Soils
Abstract
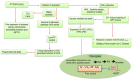
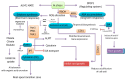
Aluminum (Al) makes up a third of the Earth's crust and is a widespread toxic contaminant, particularly in acidic soils. It impacts crops at multiple levels, from cellular to whole plant systems. This review delves into Al's reactivity, including its cellular transport, involvement in oxidative redox reactions, and development of specific metabolites, as well as the influence of genes on the production of membrane channels and transporters, alongside its role in triggering senescence. It discusses the involvement of channel proteins in calcium influx, vacuolar proton pumping, the suppression of mitochondrial respiration, and the initiation of programmed cell death. At the cellular nucleus level, the effects of Al on gene regulation through alterations in nucleic acid modifications, such as methylation and histone acetylation, are examined. In addition, this review outlines the pathways of Al-induced metabolic disruption, specifically citric acid metabolism, the regulation of proton excretion, the induction of specific transcription factors, the modulation of Al-responsive proteins, changes in citrate and nucleotide glucose transporters, and overall metal detoxification pathways in tolerant genotypes. It also considers the expression of phenolic oxidases in response to oxidative stress, their regulatory feedback on mitochondrial cytochrome proteins, and their consequences on root development. Ultimately, this review focuses on the selective metabolic pathways that facilitate Al exclusion and tolerance, emphasizing compartmentalization, antioxidative defense mechanisms, and the control of programmed cell death to manage metal toxicity.
Keywords: antioxidant defense; environmental pollution; organic acid exudation; programmed cell death; toxic metals; vacuolar processing enzymes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants.Rev Environ Contam Toxicol. 2014;232:1-44. doi: 10.1007/978-3-319-06746-9_1. Rev Environ Contam Toxicol. 2014. PMID: 24984833 Review.
-
Aluminum phytotoxicity in acidic environments: A comprehensive review of plant tolerance and adaptation strategies.Ecotoxicol Environ Saf. 2024 Jan 1;269:115791. doi: 10.1016/j.ecoenv.2023.115791. Epub 2023 Dec 8. Ecotoxicol Environ Saf. 2024. PMID: 38070417 Review.
-
Molecular and physiological strategies to increase aluminum resistance in plants.Mol Biol Rep. 2012 Mar;39(3):2069-79. doi: 10.1007/s11033-011-0954-4. Epub 2011 Jun 10. Mol Biol Rep. 2012. PMID: 21660471 Review.
-
Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize.Plant J. 2010 Mar;61(5):728-40. doi: 10.1111/j.1365-313X.2009.04103.x. Epub 2009 Dec 10. Plant J. 2010. PMID: 20003133
-
Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6503-8. doi: 10.1073/pnas.1318975111. Epub 2014 Apr 11. Proc Natl Acad Sci U S A. 2014. PMID: 24728832 Free PMC article.
Cited by
-
Silicon Nanomaterials Enhance Seedling Growth and Plant Adaptation to Acidic Soil by Promoting Photosynthesis and Antioxidant Activity in Mustard (Brassica campestris L.).Int J Mol Sci. 2024 Sep 25;25(19):10318. doi: 10.3390/ijms251910318. Int J Mol Sci. 2024. PMID: 39408646 Free PMC article.
-
Metabolic and Nutritional Responses of Contrasting Aluminium-Tolerant Banana Genotypes Under Al Stress.Plants (Basel). 2025 Jan 27;14(3):385. doi: 10.3390/plants14030385. Plants (Basel). 2025. PMID: 39942947 Free PMC article.
References
-
- Haghighi T.M., Saharkhiz M.J., Kavoosi G., Jowkar A. Monitoring amino acid profile and protein quality of licorice (Glycyrrhiza glabra L.) under drought stress, silicon nutrition and mycorrhiza inoculation. Sci. Hortic. 2022;295:110808. doi: 10.1016/j.scienta.2021.110808. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

