MaMYBR30, a Novel 1R-MYB, Plays Important Roles in Plant Development and Abiotic Stress Resistance
- PMID: 38999634
- PMCID: PMC11244220
- DOI: 10.3390/plants13131794
MaMYBR30, a Novel 1R-MYB, Plays Important Roles in Plant Development and Abiotic Stress Resistance
Abstract
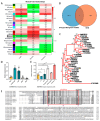
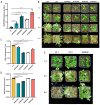
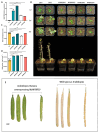
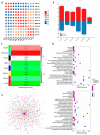
The V-myb myeloblastosis viral oncogene homolog (MYB) family participate in various bioprocesses including development and abiotic stress responses. In the present study, we first report a 1R SHAQKYF-class MYB, MaMYBR30, in mulberry. Subcellular localization and sequence analysis indicated MaMYBR30 is located in the nucleus and belongs to a CCA-like subgroup with a conserved SHAQKYF motif. Expression profile analysis showed that MaMYBR30 is expressed in leaves and can be induced by drought and salt stress. The down-regulation of MaMYBR30 using virus-induced gene silence (VIGS) in mulberry and the overexpression of MaMYBR30 in Arabidopsis were induced to explore the function of MaMYBR30. The functional characterization of MaMYBR30 in vivo indicated that MaMYBR30 can positively regulate the resistance of mulberry to drought while negatively regulating the resistance of mulberry to salt stress. In addition, MaMYBR30 also affects flower development and reproductive growth, especially after exposure to salt stress. Weighted gene co-expression network analysis (WGCNA) primarily revealed the possible genes and signal pathways that are regulated by MaMYBR30. Our results also imply that complex molecular mechanisms mediated by MaMYBR30, including crosstalk of ion toxicity, phytohormone signal transduction, flowering development, and epigenetic modification, need to be further explored in the future.
Keywords: MYB transcription factor; co-expression; drought stress; ion toxicity; salt stress.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



Similar articles
-
[MYB-like transcription factor SiMYB42 from foxtail millet (Setaria italica L.) enhances Arabidopsis tolerance to low-nitrogen stress].Yi Chuan. 2018 Apr 20;40(4):327-338. doi: 10.16288/j.yczz.17-315. Yi Chuan. 2018. PMID: 29704378 Chinese.
-
Research advances of MYB transcription factors in plant stress resistance and breeding.Plant Signal Behav. 2019;14(8):1613131. doi: 10.1080/15592324.2019.1613131. Epub 2019 May 14. Plant Signal Behav. 2019. PMID: 31084451 Free PMC article. Review.
-
Functional characterization of MaEXPA11 and its roles in response to biotic and abiotic stresses in mulberry.Plant Physiol Biochem. 2024 Jan;206:108289. doi: 10.1016/j.plaphy.2023.108289. Epub 2023 Dec 23. Plant Physiol Biochem. 2024. PMID: 38154294
-
Genome-wide characterization and expression analysis of MYB transcription factors in Chrysanthemum nankingense.BMC Plant Biol. 2023 Mar 14;23(1):140. doi: 10.1186/s12870-023-04137-7. BMC Plant Biol. 2023. PMID: 36915063 Free PMC article.
-
Multiple Functions of MYB Transcription Factors in Abiotic Stress Responses.Int J Mol Sci. 2021 Jun 7;22(11):6125. doi: 10.3390/ijms22116125. Int J Mol Sci. 2021. PMID: 34200125 Free PMC article. Review.
Cited by
-
Growth, Evapotranspiration, Gas Exchange and Chl a Fluorescence of Ipê-Rosa Seedlings at Different Levels of Water Replacement.Plants (Basel). 2024 Oct 11;13(20):2850. doi: 10.3390/plants13202850. Plants (Basel). 2024. PMID: 39458797 Free PMC article.
-
Genome-wide identification and expression profiling of MYB transcription factors in Artemisia argyi.BMC Genomics. 2025 Apr 18;26(1):384. doi: 10.1186/s12864-025-11441-z. BMC Genomics. 2025. PMID: 40251470 Free PMC article.
References
Grants and funding
- 32201526/National Natural Science Foundation of China
- 19200382/Crop Germplasm Resources Protection Project of the Ministry of Agriculture and Rural Affairs of the People's Republic of China
- NCGRC-2020-041/National Infrastructure for Crop Germplasm Resources
- CARS-18/China Agriculture Research System of MOF and MARA
LinkOut - more resources
Full Text Sources

