Blood Phenylalanine Levels in Patients with Phenylketonuria from Europe between 2012 and 2018: Is It a Changing Landscape?
- PMID: 38999811
- PMCID: PMC11243388
- DOI: 10.3390/nu16132064
Blood Phenylalanine Levels in Patients with Phenylketonuria from Europe between 2012 and 2018: Is It a Changing Landscape?
Abstract
Background: In 2011, a European phenylketonuria (PKU) survey reported that the blood phenylalanine (Phe) levels were well controlled in early life but deteriorated with age. Other studies have shown similar results across the globe. Different target blood Phe levels have been used throughout the years, and, in 2017, the European PKU guidelines defined new targets for blood Phe levels. This study aimed to evaluate blood Phe control in patients with PKU across Europe.
Methods: nine centres managing PKU in Europe and Turkey participated. Data were collected retrospectively from medical and dietetic records between 2012 and 2018 on blood Phe levels, PKU severity, and medications.
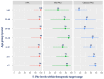
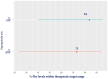
Results: A total of 1323 patients (age range:1-57, 51% male) participated. Patient numbers ranged from 59 to 320 in each centre. The most common phenotype was classical PKU (n = 625, 48%), followed by mild PKU (n = 357, 27%) and hyperphenylalaninemia (HPA) (n = 325, 25%). The mean percentage of blood Phe levels within the target range ranged from 65 ± 54% to 88 ± 49% for all centres. The percentage of Phe levels within the target range declined with increasing age (<2 years: 89%; 2-5 years: 84%; 6-12 years: 73%; 13-18 years: 85%; 19-30 years: 64%; 31-40 years: 59%; and ≥41 years: 40%). The mean blood Phe levels were significantly lower and the percentage within the target range was significantly higher (p < 0.001) in patients with HPA (290 ± 325 μmol/L; 96 ± 24%) and mild PKU (365 ± 224 μmol/L; 77 ± 36%) compared to classical PKU (458 ± 350 μmol/L, 54 ± 46%). There was no difference between males and females in the mean blood Phe levels (p = 0.939), but the percentage of Phe levels within the target range was higher in females among school-age children (6-12 years; 83% in females vs. 78% in males; p = 0.005), adolescents (13-18 years; 62% in females vs. 59% in males; p = 0.034) and adults (31-40 years; 65% in females vs. 41% in males; p < 0.001 and >41 years; 43% in females vs. 28% in males; p < 0.001). Patients treated with sapropterin (n = 222) had statistically significantly lower Phe levels compared to diet-only-treated patients (mean 391 ± 334 μmol/L; percentage within target 84 ± 39% vs. 406 ± 334 μmol/L; 73 ± 41%; p < 0.001), although a blood Phe mean difference of 15 µmol/L may not be clinically relevant. An increased frequency of blood Phe monitoring was associated with better metabolic control (p < 0.05). The mean blood Phe (% Phe levels within target) from blood Phe samples collected weekly was 271 ± 204 μmol/L, (81 ± 33%); for once every 2 weeks, it was 376 ± 262 μmol/L, (78 ± 42%); for once every 4 weeks, it was 426 ± 282 μmol/L, (71 ± 50%); and less than monthly samples, it was 534 ± 468 μmol/L, (70 ± 58%).
Conclusions: Overall, blood Phe control deteriorated with age. A higher frequency of blood sampling was associated with better blood Phe control with less variability. The severity of PKU and the available treatments and resources may impact the blood Phe control achieved by each treatment centre.
Keywords: classical PKU; hyperphenylalaninaemia; metabolic control; mild PKU; monitoring; phenylalanine; phenylketonuria; sapropterin; tyrosine.
Conflict of interest statement
A.P. received an educational grant from Cambrooke Therapeutics and Biomarin and grants from Vitaflo International, Nutricia, Merck Serono, Biomarin, Mevalia, Galen, PIAM and Applied Pharma Research to attend scientific meetings. This project is also part of A.P.’s PhD, which is funded by Vitaflo International®. A.D. received research funding from Vitaflo International and financial support from Nutricia, Mevalia and Vitaflo International to attend study days and conferences. J.C.R. was a member of the European Nutritionist Expert Panel (Biomarin) and the Advisory Board for Applied Pharma Research, Vitaflo, Synlogic, Biomarin and Nutricia and received honoraria as a speaker from APR, Merck Serono, Biomarin, Nutricia, Vitaflo, Cambrooke, PIAM and Lifediet. S.E. received research funding and financial support to attend study days and conferences from both Nutricia and Vitaflo International. C.A. received honoraria from Nutricia and Vitaflo International to attend study days and conferences. A.M. received research funding and honoraria from Nutricia, Vitaflo International and Biomarin. She is a member of the Advisory Board Element (Danone-Nutricia). F.F. was a member of the Advisory Board for Vitaflo, Biomarin, Chiesi, Sanofi-Genzyme and Nutricia and received honoraria as a speaker from Recordati, Biomarin, Merck Serono, Vitaflo, Travere Therapeutics, PTC Therapeutics, Lucane and Alexion. M.G. received financial support and honoraria as a speaker from Biomarin, Nutricia, Vitaflo, Nestlé Polska, Mead-Johnson, PTC Therapeutics International and APR Applied Pharma Research. The remaining authors do not have any conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Manti F., Nardecchia F., De Leo S., Carducci C., Romani C., Palermo L., Angeloni A., Leuzzi V. Towards precision medicine for phenylketonuria: The effect of restoring a strict metabolic control in adult patients with early-treated phenylketonuria. Mol. Genet. Metab. 2023;140:107666. doi: 10.1016/j.ymgme.2023.107666. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

