A Mediation Analysis of Obesity and Adiponectin Association with Postmenopausal Breast Cancer Risk: A Nested Cohort Study in the International Breast Cancer Intervention Study II (IBIS-II) Prevention Trial
- PMID: 38999846
- PMCID: PMC11242930
- DOI: 10.3390/nu16132098
A Mediation Analysis of Obesity and Adiponectin Association with Postmenopausal Breast Cancer Risk: A Nested Cohort Study in the International Breast Cancer Intervention Study II (IBIS-II) Prevention Trial
Abstract
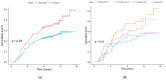
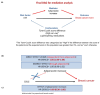
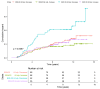
Obesity is a risk factor for postmenopausal breast cancer (BC), and evidence suggests a role for adiponectin in the relationship between obesity and BC. We investigated whether adiponectin or other biomarkers mediate the effect of body mass index (BMI) on postmenopausal BC risk in a cohort study nested in the IBIS-II Prevention Trial. We measured adiponectin, leptin, IGF-I, IGFBP-1, high-sensitivity C-reactive protein, glycemia, insulin, HOMA-IR index, and SHBG in baseline and 12-month serum samples from 123 cases and 302 matched controls in the placebo arm of the IBIS-II Prevention trial. We conducted the main mediation analysis considering baseline BMI as an exposure and the 12-month adiponectin increase as a mediator after adjustment for the Tyrer-Cuzick score and the lipid-lowering medications/supplements use. In the multivariable Cox model, both the 12-month adiponectin increase (HR, 0.60; 95%CI, 0.36-1.00) and BMI were associated with BC risk (HR, 1.05; 95%CI, 1.00-1.09), with a 40% reduction in women with a 12-month increase in adiponectin. A significantly higher cumulative hazard of BC events was observed in obese women (BMI > 30) with decreased adiponectin (p = 0.0087). No mediating effect of the adiponectin increase on the total effect of BMI on BC risk was observed (natural indirect effect: HR, 1.00; 95%CI, 0.98-1.02). Raising adiponectin levels might be an attractive target for postmenopausal BC prevention.
Keywords: BMI; adiponectin; breast cancer prevention; breast cancer risk; mediation analysis.
Conflict of interest statement
J.C. received funding for the IBIS-II study from Cancer Research UK and AstraZeneca. The funders had no role in the study design, collection, analyses, or interpretation of data, writing of the manuscript, or decision to publish the results. The other authors have no relevant financial or nonfinancial interests to disclose.
Figures
References
-
- Crew K.D., Silverman T.B., Vanegas A., Trivedi M.S., Dimond J., Mata J., Sin M., Jones T., Terry M.B., Tsai W.Y., et al. Study Protocol: Randomized Controlled Trial of Web-Based Decision Support Tools for High-Risk Women and Healthcare Providers to Increase Breast Cancer Chemoprevention. Contemp. Clin. Trials Commun. 2019;16:100433. doi: 10.1016/j.conctc.2019.100433. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

