Anti-Obesity Activity of Sanghuangporus vaninii by Inhibiting Inflammation in Mice Fed a High-Fat Diet
- PMID: 38999906
- PMCID: PMC11243596
- DOI: 10.3390/nu16132159
Anti-Obesity Activity of Sanghuangporus vaninii by Inhibiting Inflammation in Mice Fed a High-Fat Diet
Abstract
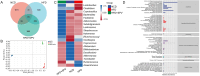
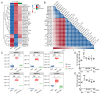
Obesity is an unhealthy condition associated with various diseases characterized by excess fat accumulation. However, in China, the prevalence of obesity is 14.1%, and it remains challenging to achieve weight loss or resolve this issue through clinical interventions. Sanghuangpours vaninii (SPV) is a nutritional fungus with multiple pharmacological activities and serves as an ideal dietary intervention for combating obesity. In this study, a long-term high-fat diet (HFD) was administered to induce obesity in mice. Different doses of SPV and the positive drug simvastatin (SV) were administered to mice to explore their potential anti-obesity effects. SPV regulated weight, serum lipids, and adipocyte size while inhibiting inflammation and hepatic steatosis. Compared with the vehicle-treated HFD-fed mice, the lowest decreases in total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) were 9.72%, 9.29%, and 12.29%, respectively, and the lowest increase in high-density lipoprotein cholesterol (HDL-C) was 5.88% after treatment with different doses of SPV. With SPV treatment, the analysis of gut microbiota and serum lipids revealed a significant association between lipids and inflammation-related factors, specifically sphingomyelin. Moreover, Western blotting results showed that SPV regulated the toll-like receptor (TLR4)/nuclear factor kappa B (NF-κB) signaling pathway in HFD-diet mice, which is related to inflammation and lipid metabolism. This research presents empirical proof of the impact of SPV therapy on obesity conditions.
Keywords: Sanghuangpours vaninii; TLR4/NF-κB; inflammation; lipids; obesity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Structural characterization and hypolipidemic activity of a hetero-galactan purified from Sanghuangporus vaninii based on modulation of TLR4/NF-κB pathway.Carbohydr Polym. 2025 Jan 1;347:122702. doi: 10.1016/j.carbpol.2024.122702. Epub 2024 Sep 5. Carbohydr Polym. 2025. PMID: 39486943
-
Inonotus hispidus Protects against Hyperlipidemia by Inhibiting Oxidative Stress and Inflammation through Nrf2/NF-κB Signaling in High Fat Diet Fed Mice.Nutrients. 2022 Aug 24;14(17):3477. doi: 10.3390/nu14173477. Nutrients. 2022. PMID: 36079733 Free PMC article.
-
Preventive Effects of Kaempferol on High-Fat Diet-Induced Obesity Complications in C57BL/6 Mice.Biomed Res Int. 2020 Apr 7;2020:4532482. doi: 10.1155/2020/4532482. eCollection 2020. Biomed Res Int. 2020. PMID: 32337249 Free PMC article.
-
Consumption of Wild Rice (Zizania latifolia) Prevents Metabolic Associated Fatty Liver Disease through the Modulation of the Gut Microbiota in Mice Model.Int J Mol Sci. 2020 Jul 29;21(15):5375. doi: 10.3390/ijms21155375. Int J Mol Sci. 2020. PMID: 32751062 Free PMC article.
-
Maternal Folic Acid Supplementation during Pregnancy Prevents Hepatic Steatosis in Male Offspring of Rat Dams Fed High-Fat Diet, Which Is Associated with the Regulation of Gut Microbiota.Nutrients. 2023 Nov 8;15(22):4726. doi: 10.3390/nu15224726. Nutrients. 2023. PMID: 38004120 Free PMC article.
Cited by
-
Metabolic-Associated Fatty Liver Disease: The Influence of Oxidative Stress, Inflammation, Mitochondrial Dysfunctions, and the Role of Polyphenols.Pharmaceuticals (Basel). 2024 Oct 10;17(10):1354. doi: 10.3390/ph17101354. Pharmaceuticals (Basel). 2024. PMID: 39458995 Free PMC article. Review.
-
Unraveling the Metabolic Pathways Between Metabolic-Associated Fatty Liver Disease (MAFLD) and Sarcopenia.Int J Mol Sci. 2025 May 14;26(10):4673. doi: 10.3390/ijms26104673. Int J Mol Sci. 2025. PMID: 40429815 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

