Submandibular Gland Pathogenesis Following SARS-CoV-2 Infection and Implications for Xerostomia
- PMID: 38999930
- PMCID: PMC11241347
- DOI: 10.3390/ijms25136820
Submandibular Gland Pathogenesis Following SARS-CoV-2 Infection and Implications for Xerostomia
Abstract
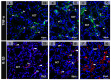
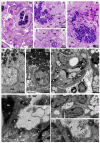
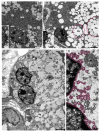
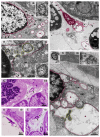
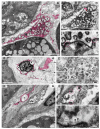
Although SARS-CoV-2 induces mucin hypersecretion in the respiratory tract, hyposalivation/xerostomia has been reported by COVID-19 patients. We evaluate the submandibular gland (SMGs) pathogenesis in SARS-CoV-2-infected K18-hACE2 mice, focusing on the impact of infection on the mucin production and structural integrity of acini, ductal system, myoepithelial cells (MECs) and telocytes. The spike protein, the nucleocapsid protein, hACE2, actin, EGF, TNF-α and IL-1β were detected by immunofluorescence, and the Egfr and Muc5b expression was evaluated. In the infected animals, significant acinar hypertrophy was observed in contrast to ductal atrophy. Nucleocapsid proteins and/or viral particles were detected in the SMG cells, mainly in the nuclear membrane-derived vesicles, confirming the nuclear role in the viral formation. The acinar cells showed intense TNF-α and IL-1β immunoexpression, and the EGF-EGFR signaling increased, together with Muc5b upregulation. This finding explains mucin hypersecretion and acinar hypertrophy, which compress the ducts. Dying MECs and actin reduction were also observed, indicating failure of contraction and acinar support, favoring acinar hypertrophy. Viral assembly was found in the dying telocytes, pointing to these intercommunicating cells as viral transmitters in SMGs. Therefore, EGF-EGFR-induced mucin hypersecretion was triggered by SARS-CoV-2 in acinar cells, likely mediated by cytokines. The damage to telocytes and MECs may have favored the acinar hypertrophy, leading to ductal obstruction, explaining xerostomia in COVID-19 patients. Thus, acinar cells, telocytes and MECs may be viral targets, which favor replication and cell-to-cell viral transmission in the SMG, corroborating the high viral load in saliva of infected individuals.
Keywords: DMV; Sjögren’s syndrome; acinar cell; immunofluorescence; mucin; myoepithelial cell; salivary gland; sialadenosis; telocytes; transmission electron microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Aberrant MUC1 accumulation in salivary glands of Sjögren's syndrome patients is reversed by TUDCA in vitro.Rheumatology (Oxford). 2020 Apr 1;59(4):742-753. doi: 10.1093/rheumatology/kez316. Rheumatology (Oxford). 2020. PMID: 31377809
-
Epiregulin is critical for the acinar cell regeneration of the submandibular gland in a mouse duct ligation model.J Oral Pathol Med. 2014 May;43(5):378-87. doi: 10.1111/jop.12145. Epub 2013 Dec 20. J Oral Pathol Med. 2014. PMID: 24354788
-
SARS-CoV-2 exploits steroidogenic machinery, triggers lipid metabolism for viral replication and induces immune response in Leydig cells of K18-hACE2 mice.Front Cell Infect Microbiol. 2025 May 27;15:1538461. doi: 10.3389/fcimb.2025.1538461. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40496014 Free PMC article.
-
Covid-19 and oral diseases: Crosstalk, synergy or association?Rev Med Virol. 2021 Nov;31(6):e2226. doi: 10.1002/rmv.2226. Epub 2021 Mar 1. Rev Med Virol. 2021. PMID: 33646645 Free PMC article. Review.
-
Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis.Clin Microbiol Rev. 2021 May 12;34(3):e00299-20. doi: 10.1128/CMR.00299-20. Print 2021 Jun 16. Clin Microbiol Rev. 2021. PMID: 33980688 Free PMC article. Review.
Cited by
-
Telocytes: history, origin, identification, structure, distribution, and functions.Histochem Cell Biol. 2025 Aug 29;163(1):86. doi: 10.1007/s00418-025-02413-1. Histochem Cell Biol. 2025. PMID: 40883527 Review.
-
Characteristics of Oral Adverse Effects following COVID-19 Vaccination and Similarities with Oral Symptoms in COVID-19 Patients: Taste and Saliva Secretory Disorders.Med Princ Pract. 2025;34(2):101-120. doi: 10.1159/000543182. Epub 2024 Dec 19. Med Princ Pract. 2025. PMID: 39701050 Free PMC article. Review.
References
-
- Matuck B.F., Dolhnikoff M., Duarte-Neto A.N., Maia G., Gomes S.C., Sendyk D.I., Zarpellon A., de Andrade N.P., Monteiro R.A., Pinho J.R.R., et al. Salivary glands are a target for SARS-CoV-2: A source for saliva contamination. J. Pathol. 2021;254:239–243. doi: 10.1002/path.5679. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

